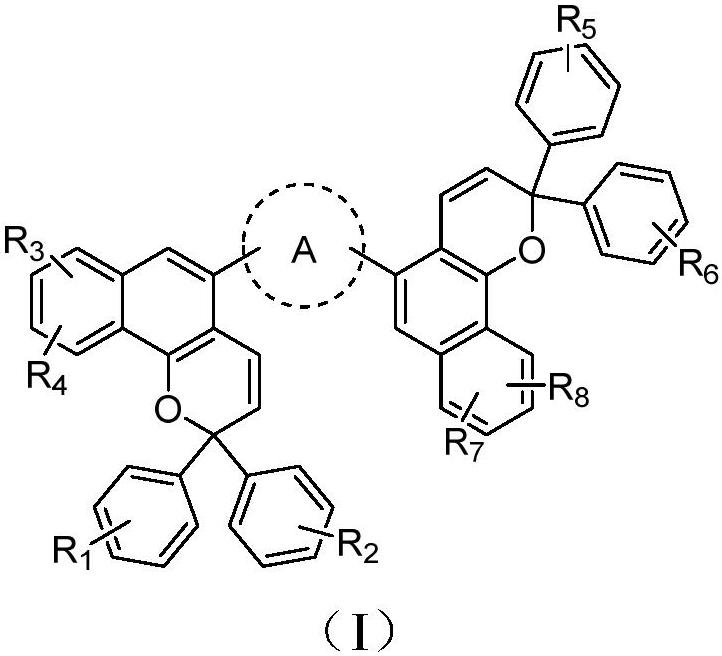
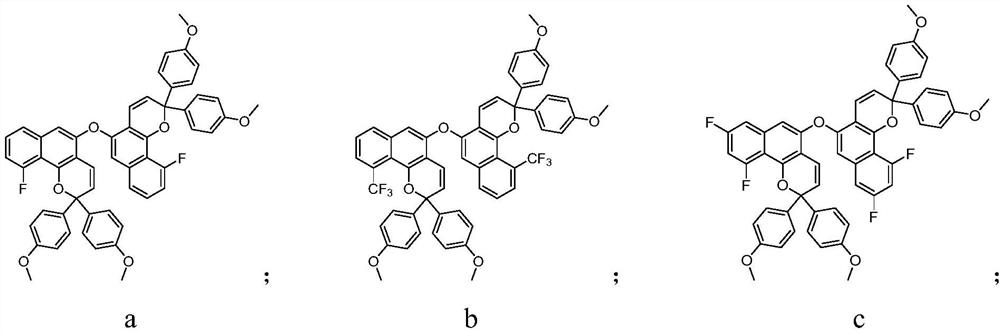
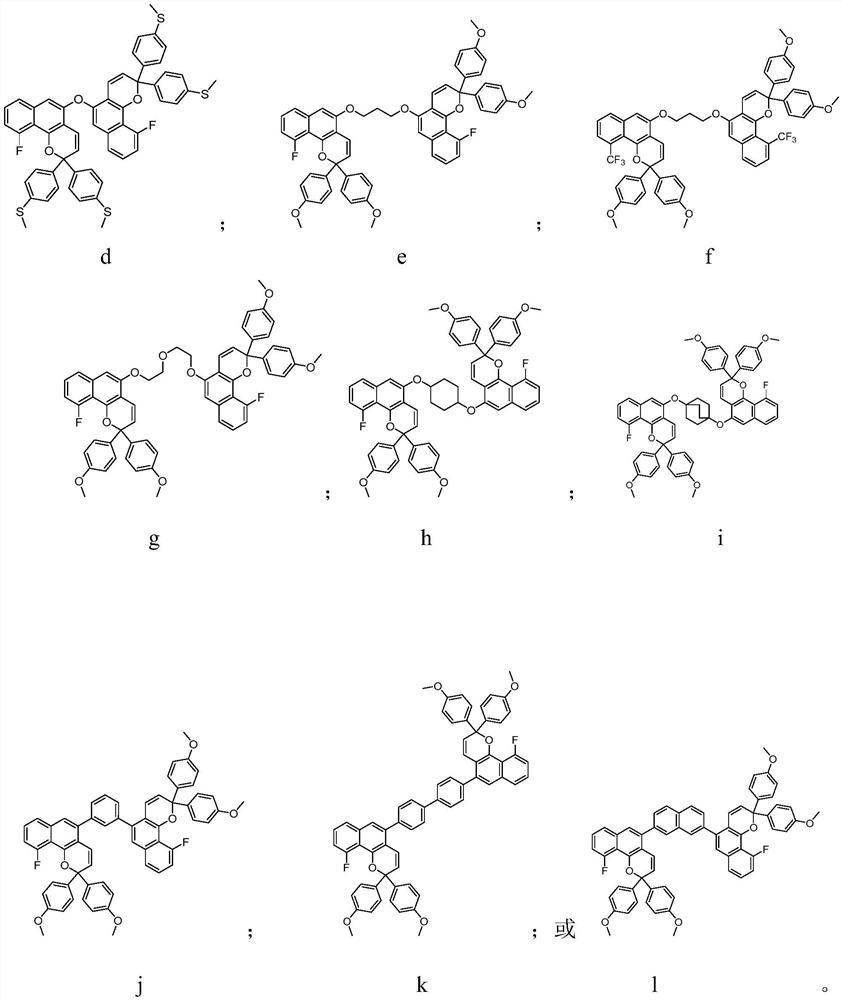
Photochromic compound and application thereof
A photochromic and photochromic material technology, applied in the direction of color-changing fluorescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of lens color change, different anti-UV aging characteristics, etc., and achieve high color sensitivity and excellent color rendering Durability, Effect of Short Fade Half-Life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Preparation of intermediates:
[0054] 1. Preparation of intermediate A-5: 10-fluoro-2,2-bis(4-methoxyphenyl)-2H-benzo[h]chromen-5-ol
[0055]
[0056] (1) A-3: Preparation of 1,1-bis(4-methoxyphenyl)prop-2-yn-1-ol
[0057] 4,4'-dimethoxybenzophenone A-1 (500mg, 2.06mmol) was dissolved in 10mL of ethylenediamine, and acetylene lithium ethylenediamine complex A-2 (558mg, 6.20mmol) was added. Stir at room temperature for 2 hours under nitrogen atmosphere. After the reaction was completed, it was quenched with ice water, and the reaction solution was extracted with ethyl acetate. The organic layer was washed with water and saturated sodium chloride, and dried over anhydrous sodium sulfate. After concentration, the crude product was purified by silica gel column chromatography to obtain 1,1-bis(4-methoxyphenyl)prop-2-yn-1-ol A-3 (450 mg, white solid), yield: 81%. ESI-MS m / z: 269[M+H] + .
[0058] (2) A-5: Preparation of 10-fluoro-2,2-bis(4-methoxyphenyl)-2H-benzo[h...
Embodiment 1
[0073] Example 1: Preparation of 5,5'-oxybis(10-fluoro-2,2-bis(4-methoxyphenyl)-2H-benzo[h]chromene)
[0074]
[0075] 10-Fluoro-2,2-bis(4-methoxyphenyl)-2H-benzo[h]chromen-5-ol A-5 (200 mg, 0.47 mmol) was dissolved in tetrahydrofuran (5 mL), Add diethyl azodicarboxylate (DEAD, 35mg, 0.2mmol) and triphenylphosphine (52mg, 0.2mmol) and react for 16 hours. After the reaction is completed, concentrate and separate through silica gel preparation plate to obtain 5,5'-oxo Bis(10-fluoro-2,2-bis(4-methoxyphenyl)-2H-benzo[h]chromene) (75 mg, white solid), yield: 38%. ESI-MS m / z: 839[M+H] + .
[0076] 1 H-NMR (400MHz, DMSO-d 6 ): δ7.83-7.62(m, 2H), 7.47-7.32(m, 10H), 7.30-7.15(m, 2H), 6.88-6.70(m, 10H), 6.58(d, J=7.2Hz, 2H ), 6.39 (d, J=7.2Hz, 2H), 3.52 (s, 12H).
Embodiment 2
[0077] Example 2: Preparation of 5,5'-oxybis(10-trifluoromethyl-2,2-bis(4-methoxyphenyl)-2H-benzo[h]chromene)
[0078]
[0079] 10-Trifluoromethyl-2,2-bis(4-methoxyphenyl)-2H-benzo[h]chromen-5-ol A-7 (200 mg, 0.42 mmol) was dissolved in tetrahydrofuran (5 mL ), add diethyl azodicarboxylate (DEAD, 31mg, 0.18mmol) and triphenylphosphine (47mg, 0.18mmol) and react for 16 hours. '-Oxybis(10-trifluoromethyl-2,2-bis(4-methoxyphenyl)-2H-benzo[h]chromene) (50 mg, white solid), yield: 23%. ESI-MSm / z: 939[M+H] + .
[0080] 1 H-NMR (400MHz, DMSO-d 6 ): δ8.12-7.65 (m, 2H), 7.55-7.37 (m, 10H), 7.32-7.12 (m, 2H), 6.89-6.72 (m, 10H), 6.57 (d, J=7.2Hz, 2H ), 6.41 (d, J=7.2Hz, 2H), 3.55 (s, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com