Preparation method for co-production of cyclododecene ether and cyclododecanol
A technology of cyclododecene ether and cyclododecanol is applied in the preparation field of co-production of cyclododecene ether and cyclododecanol, and can solve the problem of increased input cost of basic substances, high peroxide concentration, and oxidizing agent. High cost problem, to achieve the effect of improving technological innovation and process competitiveness, less by-products, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
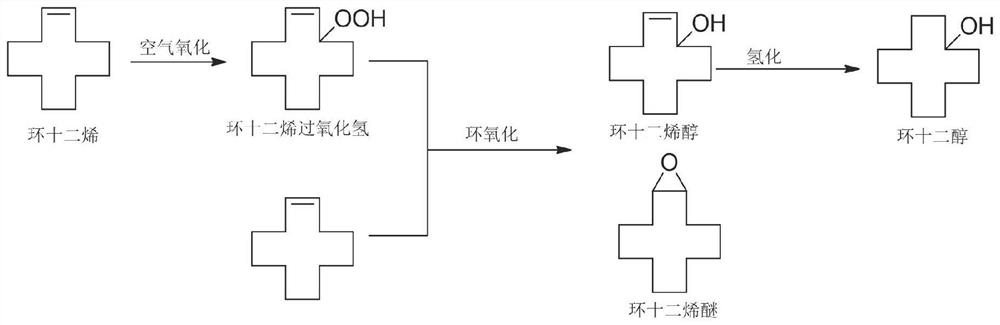
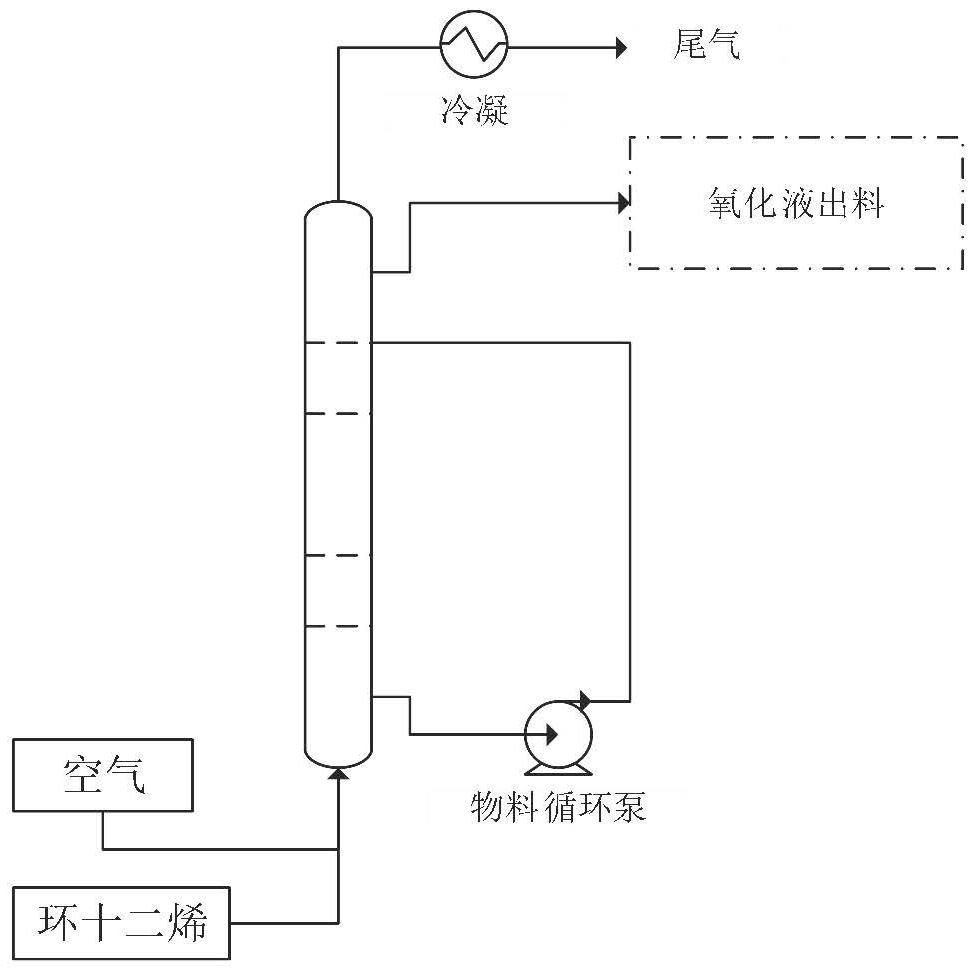
[0032] This embodiment combines figure 1 and figure 2 A preparation method for the co-production of cyclododecenyl ether and cyclododecanol in the present invention is described.
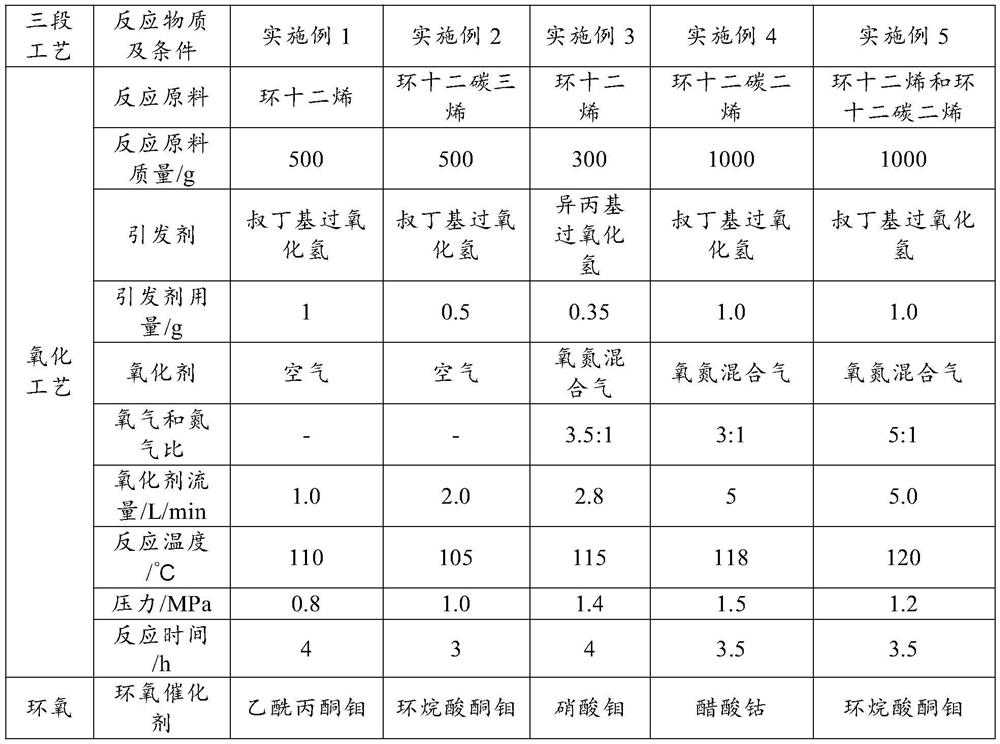
[0033] Add 500 grams of cyclododecene into a 2L autoclave. Add 1.0 g of tert-butyl hydroperoxide initiator; feed air at a flow rate of 1.0 L / min. The reaction temperature is 110°C, the pressure is 0.8MPa, and the residence time is 4h.
[0034] Add 0.1 g of molybdenum acetylacetonate to the mixed solution. The reaction temperature is 150° C., the pressure is 0.3 MPa, and the reaction time is 45 minutes.
[0035] After removing unreacted raw materials and catalysts from the above mixed solution, 129.5 grams of the mixed solution was added to a 500 mL autoclave. Add 10% Ni-SiO to the mixture 2 Catalyst 0.3 g. The reaction temperature is 120° C., the hydrogen pressure is 6 MPa, and the reaction time is 3 h. The finished product is 125.6 grams in total, the ratio of cyclododecenyl ether to cyclo...
Embodiment 2
[0037]Add 500 grams of cyclododecatriene into a 2L autoclave. Add 0.5 g of tert-butyl hydroperoxide initiator; feed air at a flow rate of 2.0 L / min. The reaction temperature is 105° C., the pressure is 1.0 MPa, and the residence time is 3 h.
[0038] Add 1.5 grams of molybdenum naphthenate ketone in the mixed solution. The reaction temperature is 130° C., the pressure is 0.4 MPa, and the reaction time is 35 minutes.
[0039] After removing unreacted raw materials and catalysts from the above mixed solution, 144.6 grams of the mixed solution was added to a 500 mL autoclave. Add 40% Ni-SiO to the mixture 2 Catalyst 0.5 g. The reaction temperature is 110° C., the hydrogen pressure is 7 MPa, and the reaction time is 1 h. The finished product is 132.5 grams in total, the ratio of cyclododecenyl ether to cyclododecanol is 46.2:53.8, and the total yield is 91.6%.
Embodiment 3
[0041] Add 300 grams of cyclododecene into a 1L autoclave. Add 0.35 g of isopropyl hydroperoxide (85%) initiator; feed oxygen and nitrogen mixed gas (volume ratio 3.5:1), flow rate is 2.8 L / min. The reaction temperature is 115°C, the pressure is 1.4MPa, and the residence time is 4h.
[0042] Add 2.5 grams of molybdenum nitrate in the mixed solution. The reaction temperature is 115° C., the pressure is 0.35 MPa, and the reaction time is 25 minutes.
[0043] After removing the raw material and the catalyst from the above mixed solution, 34.5 grams of the mixed solution was added into a 100 mL autoclave. Add 0.2 g of 60% Ni-alumina catalyst to the mixed solution. The reaction temperature is 110° C., the hydrogen pressure is 8 MPa, and the reaction time is 2.0 h. The finished product is 32.09 grams in total, the ratio of cyclododecenyl ether to cyclododecanol is 57.8:42.2, and the total yield is 91.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com