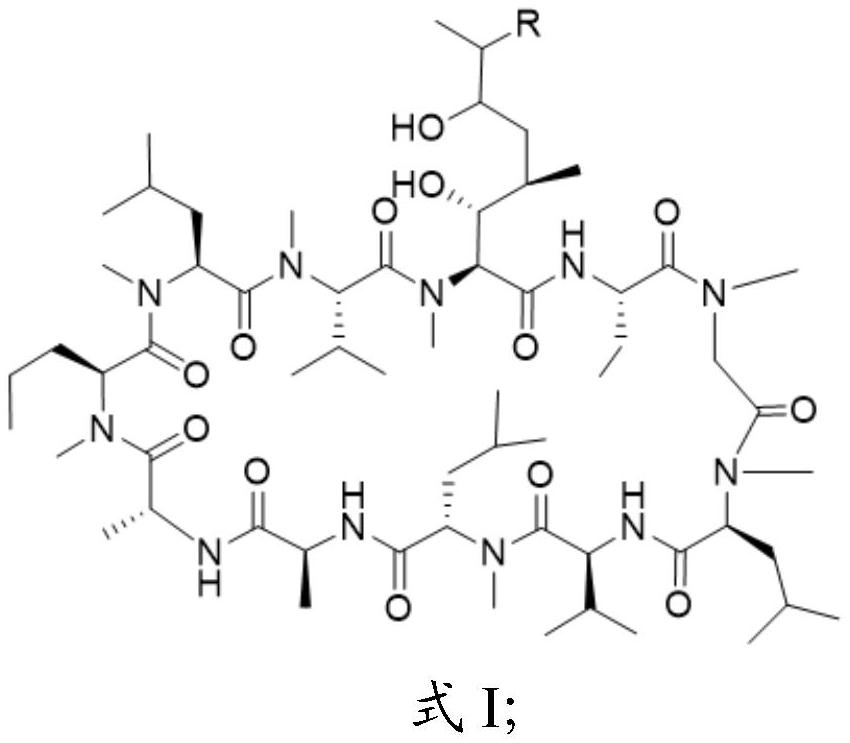
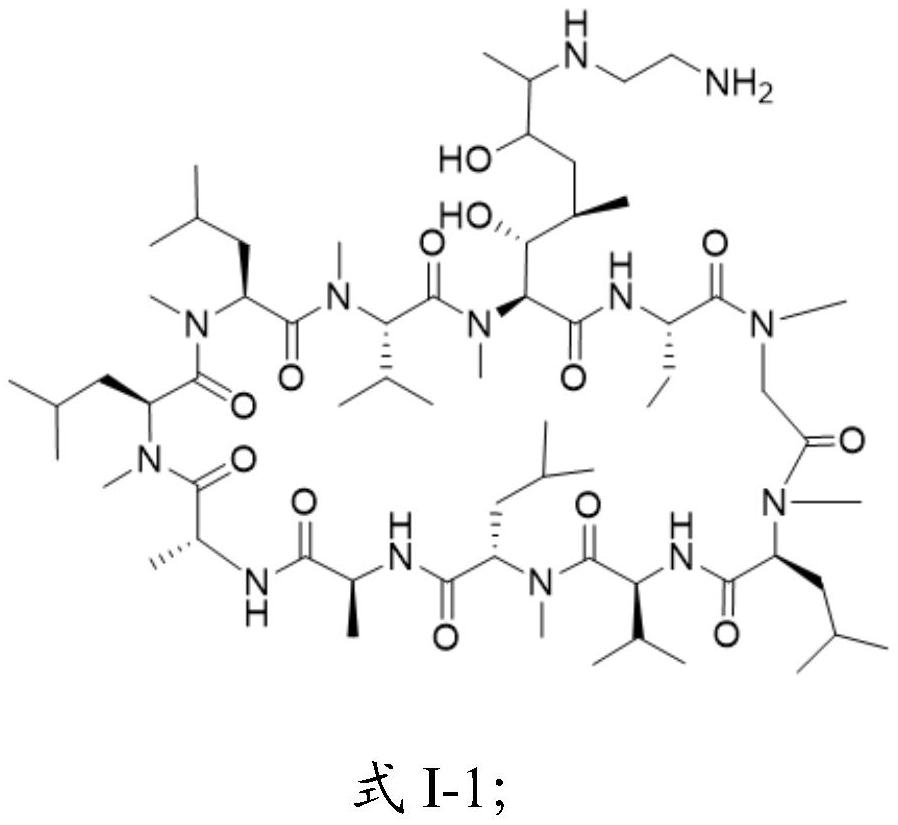
Cyclosporine A derivative as well as preparation method and application thereof
A technology for cyclosporine and derivatives, which is applied in the field of cyclosporine A derivatives and their preparation, can solve the problems of unsatisfactory yield of hydroxyl modification process, unfavorable industrial production and application, inability to obtain target substances, etc. Overcome harsh preparation conditions, high accuracy and good crystal state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of CsA ethylene oxide product
[0041]
[0042] Add CsA raw material (5.00g, 4.16mmol) into a 500mL eggplant-shaped bottle, dissolve it in 150mL of dichloromethane, add potassium peroxymonosulfonate (the molar ratio of addition to CsA is 1:1~1:15), at room temperature Stir overnight. Wash the reaction mother liquor twice with 200mL of 20% sodium sulfite solution, and wash twice with 200mL of 10% sodium carbonate aqueous solution. The organic layer was then dried over anhydrous sodium sulfate. Concentration under reduced pressure gave 4.83 g of a white solid product with a purity of 96.8% and a yield of 92.2%.
[0043] Gained CsA epoxidation product mass spectrum and proton nuclear magnetic spectrum data are as follows:
[0044] MS-ESI(m / z):1240.73[M+Na] + ;
[0045] 1 H NMR (400MHz, DMSO-d 6 )δ:7.43-7.48(m,4H,-NH-CO-),5.28(s,1H,-OH),4.61-4.36(m,11H,-CH),4.05(s,2H,-CH 2 ),3.32-2.94(m,21H,-CH3 ),2.73-2.46(m,4H,-CH),2.30-2.17(m,10H,-CH 2 )...
Embodiment 2
[0046] Embodiment 2: Preparation of CsA aminated derivative 1
[0047]
[0048] Under the protection of argon, add CsA epoxide (500.0mg, 0.41mmol) to 50ml of anhydrous tetrahydrofuran, then add ethylenediamine (the molar ratio of addition to CsA epoxide is 1:2~1:50), heat to reflux 8h~40h. After the reaction was stopped, the solvent was concentrated by a rotary evaporator under reduced pressure, then ethyl acetate was added to dissolve the remaining solid, and then washed with 0.01M dilute hydrochloric acid solution for 1 to 5 times, and 0.1M ammonia water for 1 to 3 times. The organic phase was dried with a desiccant, concentrated under reduced pressure by a rotary evaporator to remove the solvent, then added a small amount of methanol, and then added a certain amount of purified water (the volume ratio of purified water to methanol was 1:10 to 1:100) for recrystallization and purification. After filtration and drying, 468.3 mg of the white crystal derivative was obtained...
Embodiment 3
[0052] Embodiment 3: Preparation of CsA aminated derivative 2
[0053]
[0054] Under the protection of argon, add CsA epoxide (500.0mg, 0.41mmol) to 50ml of anhydrous tetrahydrofuran, and then add 1,7-heptanediamine (the molar ratio of addition to CsA epoxide is 1:2~1:50 ), heated to reflux for 8h to 40h. After the reaction was stopped, the solvent was concentrated by a rotary evaporator under reduced pressure, then ethyl acetate was added to dissolve the remaining solid, and then washed with 0.01M dilute hydrochloric acid solution for 1 to 5 times, and 0.1M ammonia water for 1 to 3 times. The organic phase was dried with a desiccant, concentrated under reduced pressure by a rotary evaporator to remove the solvent, then added a small amount of methanol, and then added a certain amount of purified water (the volume ratio of purified water to methanol was 1:10 to 1:100) for recrystallization and purification. After filtration and drying, 376.9 mg of the white crystal deriva...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com