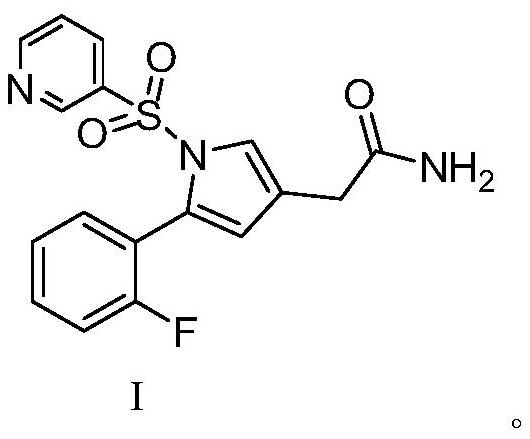
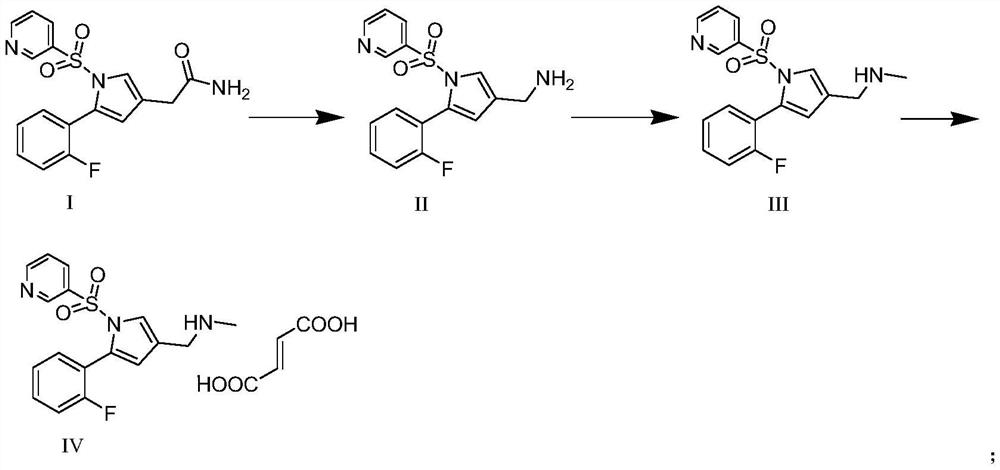
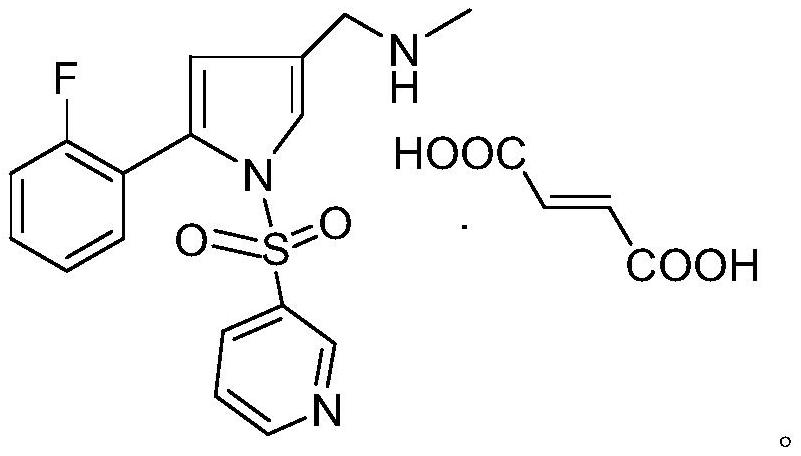
Intermediate compound for preparing vonoprazan
A technology for fumaric acid vonoprazan and compound, which is applied in the field of fumaric acid vonoprazan intermediate compound and its preparation, can solve the problems of impurity residue, cumbersome hydrogenation reduction in the operation process, etc., and achieve the reduction of impurity content and the improvement of material Easy to obtain, the effect of improving the total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 formula I compound
[0046] Weigh 39.5 g of 5-(2-fluorophenyl)-3-bromomethyl 1-(3-pyridylsulfonyl)-1H-pyrrole, add it to 120 ml of methanol, then add 5.88 g of NaCN, and heat up to 50 ℃, heat preservation reaction for 2.0h, after the reaction is completed, add an appropriate amount of sodium thiosulfate, stir for 0.5h, then cool down to 0-10°C, start to add 360ml of water dropwise, solids are formed during the dropwise addition, after the dropwise addition, pump After filtration and vacuum drying, 30.08 g of solid 5-(2-fluorophenyl)-1-(3-pyridylsulfonyl)-1H-pyrrole-3-acetonitrile was obtained, with a yield of 88.16%.
[0047]Dissolve 30.08g of 5-(2-fluorophenyl)-1-(3-pyridylsulfonyl)-1H-pyrrole-3-acetonitrile in 100mL of tetrahydrofuran solution, add 6g of 20% NaOH aqueous solution, and heat up to 55°C. Add 12.0g of 30% hydrogen peroxide solution dropwise. After the dropwise addition, keep the temperature for 1h. After the reaction, cool...
Embodiment 2
[0050] The preparation of embodiment 2 fumaric acid vonoprazan (formula IV)
[0051] Dissolve 7.57g NaOH in 15ml water, then stir and cool down to 0°C, add 19.01g Br 2 , kept stirring for 1.0h, then added dropwise the acetonitrile solution of 5-(2-fluorophenyl)-1-(3-pyridylsulfonyl)-1H-pyrrole-3-acetamide (intermediate I 34.0g , acetonitrile solution 100ml), stirred at room temperature for 0.5h after the dropwise addition, then raised the temperature to 80°C, kept stirring for 1.0h, adjusted the pH to 1-2 with hydrochloric acid after the reaction, stirred for 0.5h, and then adjusted the pH to 11. Suction filtration, the filtrate was concentrated under reduced pressure and then recrystallized to obtain 28.51 g of solid, with a yield of 90.04%.
[0052] 28.51g of 5-(2-fluorophenyl)-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methanamine was dissolved in 200ml dimethyl sulfoxide solution, 20.26g potassium carbonate was added, 15.37 g of dimethyl carbonate, heated to 120°C, kept at reflu...
Embodiment 3
[0055] The preparation of embodiment 3 vonoprazan fumarate (formula IV)
[0056] Dissolve 5.68g NaOH in 15ml water, then stir and cool down to 0°C, add 15.84g Br 2 , kept stirring for 1.0h, then added dropwise the acetonitrile solution of 5-(2-fluorophenyl)-1-(3-pyridylsulfonyl)-1H-pyrrole-3-acetamide (intermediate I 34.0g , acetonitrile solution 70ml), stirred at room temperature for 0.5h after the dropwise addition, then raised the temperature to 80°C, kept stirring for 1.0h, adjusted the pH to 1-2 with hydrochloric acid after the reaction, stirred for 0.5h, and then adjusted the pH to 11. Suction filtration, the filtrate was concentrated under reduced pressure and then recrystallized to obtain 27.28 g of solid, with a yield of 86.16%.
[0057] 27.28g of 5-(2-fluorophenyl)-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methanamine was dissolved in 300ml dimethylsulfoxide solution, 11.91g potassium carbonate was added, 7.69 g of dimethyl carbonate, heated to 120°C, kept at reflux for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com