Preparation method of noble metal doped bimetallic phosphide catalyst for electrochemical complete water splitting
A bimetal and phosphide technology, applied in the field of electrochemistry, can solve problems such as low activity, and achieve the effects of simple preparation method, good application prospect and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
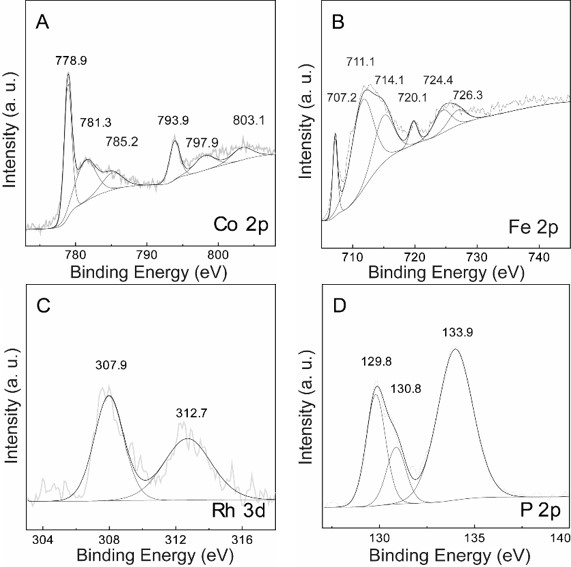
[0049] This preparation is used to illustrate a preparation method of a noble metal-doped double metal phosphide material for electrocatalytic total water splitting. The method includes:
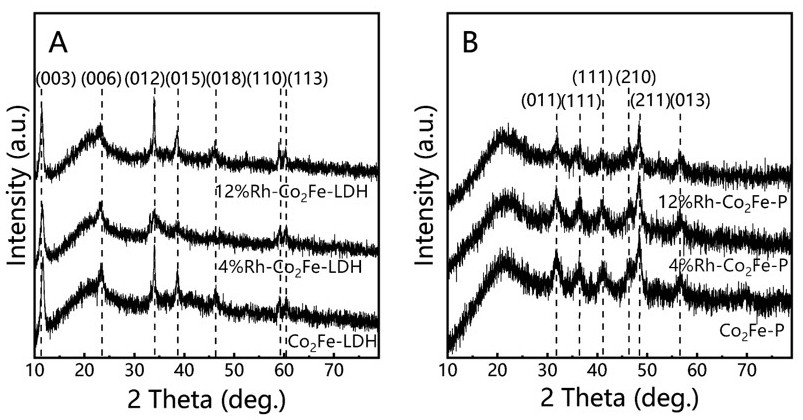
[0050] Step 101, using ethylene glycol-assisted hydrothermal method to prepare rhodium-doped cobalt-iron double hydroxide;
[0051] Step 102, annealing the rhodium-doped cobalt-iron double hydroxide and sodium hypophosphite in nitrogen to obtain the noble metal-doped double metal phosphide material.
[0052] Specifically, the preparation of rhodium-doped cobalt-iron double hydroxide by ethylene glycol-assisted hydrothermal method in step 101 includes:
[0053] Dissolving soluble cobalt salt, soluble iron salt, soluble rhodium salt and urea in a solvent according to a certain molar ratio to obtain a solution, the solvent being a mixed solvent of ethylene glycol and water at a certain volume ratio;
[0054] After the solution was stirred at room temperature for 30 min, it was poured into a po...
Embodiment 1
[0058] Embodiment 1: the cobalt-iron phosphide doped with rhodium that the ratio of cobalt-iron-rhodium is 2:1:0.1 is prepared
[0059] (1) Dissolve 0.291 g cobalt nitrate hexahydrate, 0.202 g ferric nitrate nonahydrate, 10.46 mg rhodium trichloride and 120 mg urea into 10 ml deionized water and 30 ml ethylene glycol mixed solvent, and stir for 30 minutes;
[0060] (2) Pour the above mixed solution into the polytetrafluoroethylene lining, and place the lining in a stainless steel hydrothermal kettle, and conduct a hydrothermal treatment at 120°C for 12 h;
[0061] (3) After cooling to room temperature, the product is centrifuged, washed with water and freeze-dried to obtain the rhodium-doped cobalt-iron double hydroxide;
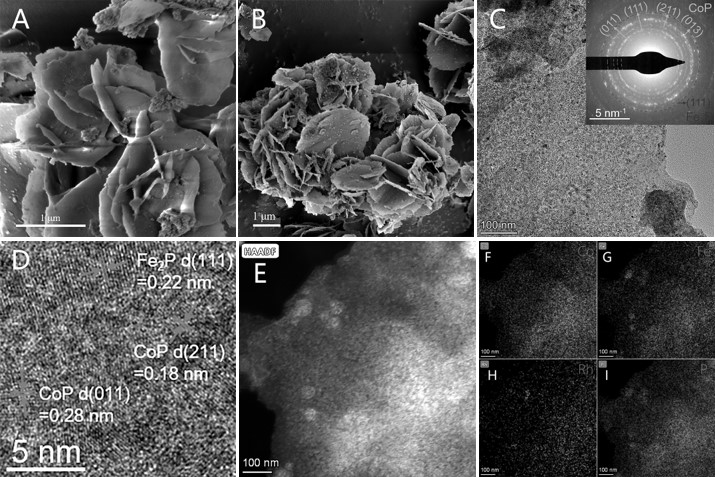
[0062] (4) Put 10 mg of rhodium-doped cobalt-iron double hydroxide obtained in (3) into a porcelain boat, put 100 mg of sodium hypophosphite into another porcelain boat upstream of the horizontal tube furnace, and place the material in Annealing at 350° C. ...
Embodiment 2
[0063] Embodiment 2: the cobalt iron phosphide that the ratio of preparation cobalt iron rhodium is 2:1:0.3 doped with rhodium
[0064] (1) Dissolve 0.291 g cobalt nitrate hexahydrate, 0.202 g ferric nitrate nonahydrate, 31.38 mg rhodium trichloride and 120 mg urea into 10 ml deionized water and 30 ml ethylene glycol mixed solvent, and stir for 30 minutes;
[0065] (2) Pour the above mixed solution into the polytetrafluoroethylene lining, and place the lining in a stainless steel hydrothermal kettle, and conduct a hydrothermal treatment at 120°C for 12 h;
[0066] (3) After cooling to room temperature, the product is centrifuged, washed with water and freeze-dried to obtain the rhodium-doped cobalt-iron double hydroxide;
[0067] (4) Put 10 mg of rhodium-doped cobalt-iron double hydroxide obtained in (3) into a porcelain boat, put 100 mg of sodium hypophosphite into another porcelain boat upstream of the horizontal tube furnace, and place the material in Annealing at 350°C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com