Preparation method of methionine
A technology of methionine and methionine amide, which is applied in the chemical industry, can solve the problems of unstable methionine yield, difficulty in efficient separation, unstable yield, etc., and achieve the effects of long recyclable cycle, shortened process flow, and good anti-pollution ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 (amination, cyanohydrin: ammonia, 1:5)
[0039] Transfer 151.76g of cyanohydrin (86.4w%, 1mol) into a 1L zirconium material 316L autoclave, add 340g of ammonia water (25w%, 5mol), seal the reactor, start heating and stirring, heat up to 50°C, and the pressure is 0.4MPa , heat preservation reaction for 45 minutes, the conversion rate of cyanohydrin in the reaction solution was detected by HPLC to reach 99.5%, and the system was cooled to room temperature to obtain 2-amino-4-methylthiobutyronitrile material, which was used for the subsequent catalytic preparation of methionine.
Embodiment 2
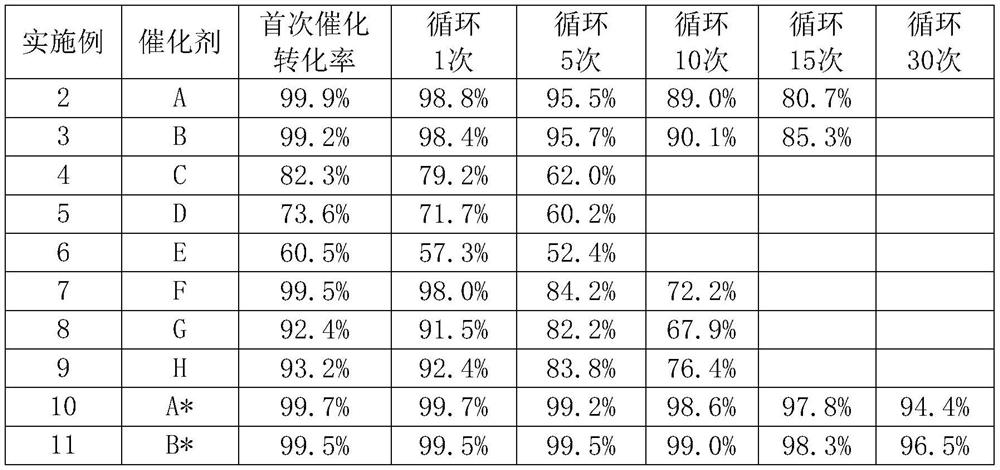
[0041] The 369.3g 2-amino-4-methylthiobutyronitrile material (35.2w%) prepared by the method in Example 1 and the 200g hydration stream were added in a 316L zirconium material autoclave, and 172g of catalyst A cerium dioxide (rod-shaped dioxide Cerium, particle size 10nm, length 100nm, surface area 86.3m 2 / g) (1.0eq), stirred at 80° C. for 1 h, filtered to remove catalyst A, analyzed the reaction solution by HPLC, and the conversion rate of 2-amino-4-methylthiobutyronitrile to methionine and its ammonium salt was 99.9%. Catalyst A was reused to catalyze the preparation of methionine from 2-amino-4-methylthiobutyronitrile, and the conversion rate changes after 15 cycles were analyzed and recorded, as shown in Table 1.
Embodiment 3
[0043] Operation is the same as in Example 2, and the catalyst is replaced by catalyst B (rod-shaped ceria, particle diameter 30nm, long 200nm, surface area 45m 2 / g). Catalyst B was recycled and catalyzed 15 times, and the changes in the conversion rate during the analysis and recording cycle were shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com