High-throughput screening method of signal peptide library based on fluorescent probe Rho-IDA-CoII
A technology of rho-ida-coii and fluorescent probes, applied in the field of high-throughput screening, to achieve the effect of ensuring diversity and avoiding loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation and plasmid transformation of Bacillus subtilis WB800 competent cells
[0045] 1) Streak the strain of Bacillus subtilis WB800 frozen at -80 °C on an antibiotic-free LB plate, and incubate at a constant temperature of 37 °C for 2 days to activate the strain;
[0046] 2) Pick a single colony and inoculate it in 5 mL of GMI solution, shake it at 30 °C and 100 rpm overnight;
[0047] 3) Transfer 2 mL of the overnight culture to 18 mL of GMI solution, incubate at 37˚C, 200 rpm for 3.5 h (determine OD every 30 min 600 , draw the curve of OD value versus time, culture to late logarithmic period);
[0048] 4) Take 10 mL of Bacillus subtilis culture medium in the late logarithmic period and transfer it into 90 mL of GM II solution, incubate at 37 °C and 100 rpm for 90 min, and then collect the cells (cultivate to the late logarithmic period);
[0049] 5) Use 2 mL of GM II solution (containing 10% glycerol) to suspend the bacteria to prepare Bacillus subti...
Embodiment 2
[0053] Example 2: Construction of a signal peptide library for secretion and expression of nitrile hydratase
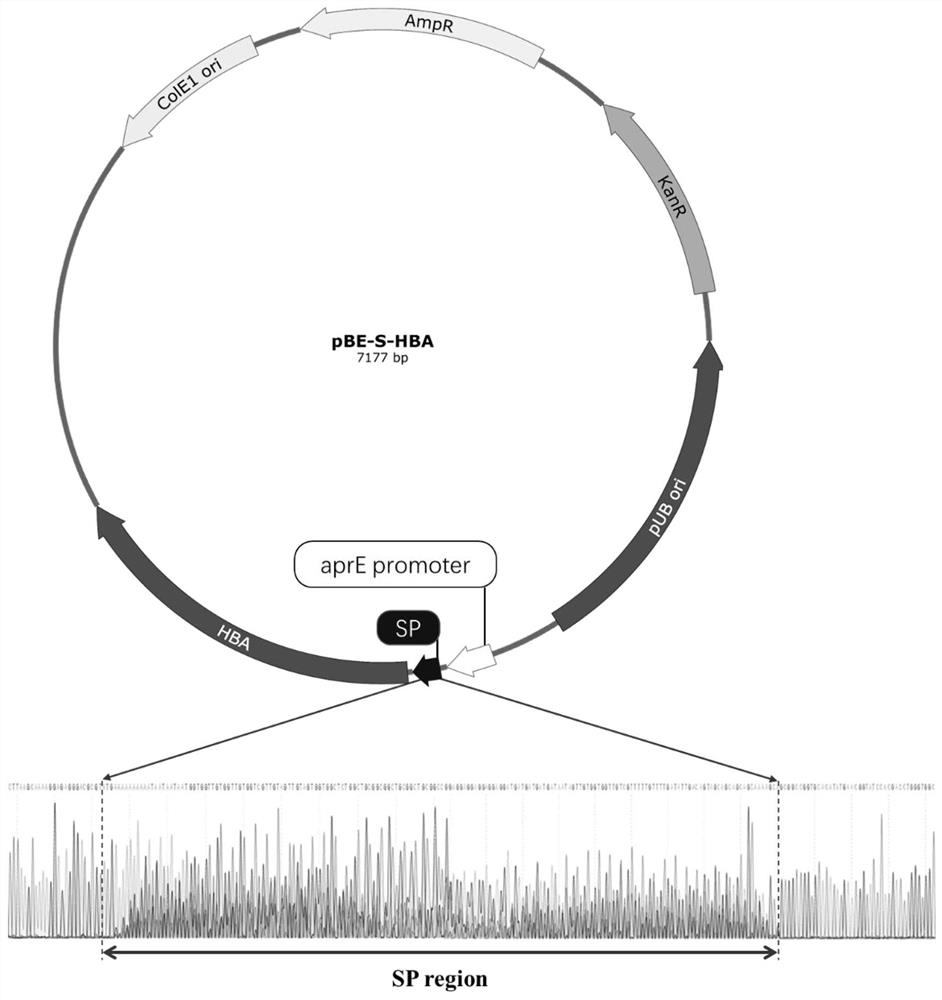
[0054] 1) According to the instructions of Axygen's plasmid mini-extraction kit, from recombinant Escherichia coli Escherichia coli The recombinant plasmid pBE-S-HBA was extracted from DH5α-pBE-S-HBA. The nucleotide sequence of wild-type nitrile hydratase HBA is shown in SEQ ID NO. 2.
[0055] 2) Design PCR vector linearization primers 5'-CGCGTCCCTCTCCCTTTTGCTTAAGTTCAGAGTAG-3' (SEQ ID NO. 3) and 5'- GGCCGGTGCACATATGAAGGACAACAACAAAGTT-3' (SEQ ID NO. 4) Using the plasmid pBE-S as a template, pre-denature at 95°C for 3 minutes according to the program; 30 cycles (denaturation at 95°C for 15 s, annealing at 68°C for 15 s, extension at 72°C for 3 min); final extension at 72°C for 5 min for PCR amplification Preparation of linearized plasmid pBE-S-HBA containing specific ends L ;
[0056] 3) According to the instructions of the ClonExpress Ultra One Step Cloning Kit kit...
Embodiment 3
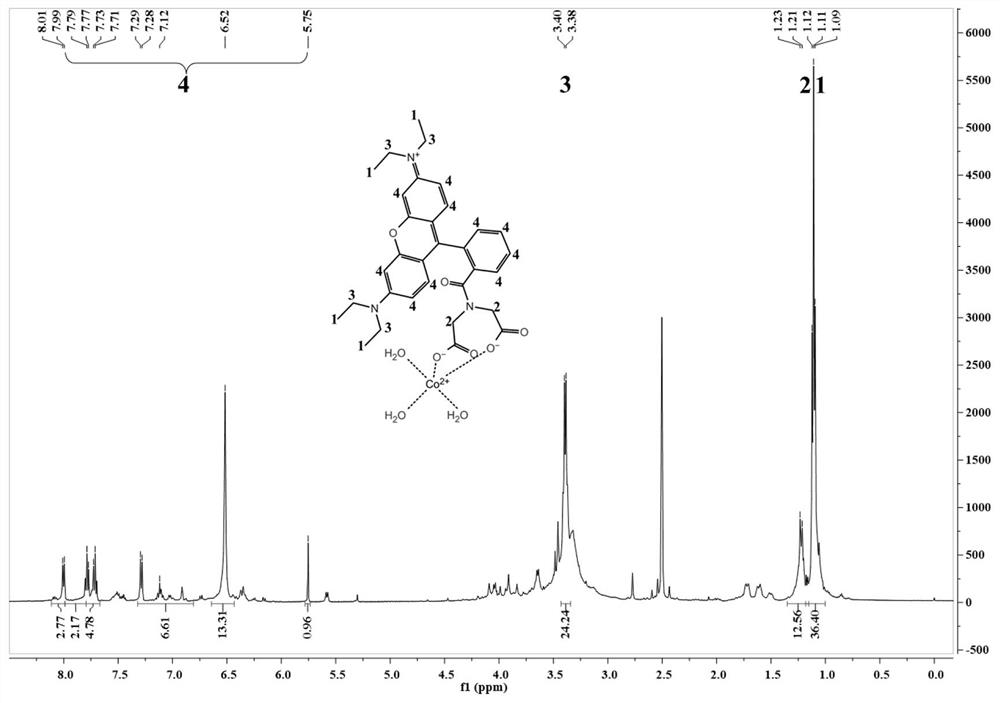
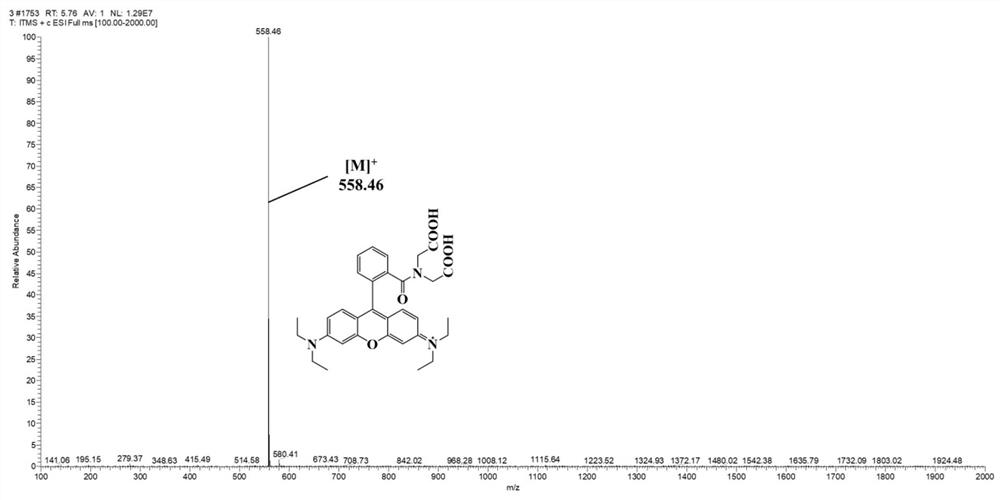
[0061] Embodiment 3: the chemical synthesis of fluorescent probe Rho-IDA
[0062] 1) In a 50 mL reaction flask equipped with a magnetic stirrer, add 0.1 mmol of rhodamine B and 10 mL of dichloromethane, and fully dissolve.
[0063] 2) Slowly add 0.15 mmol N-hydroxysuccinimide and 0.15 mmol N, N'-dicyclohexylcarbodiimide, react at room temperature overnight, and filter to remove the precipitate.
[0064] 3) Slowly add 0.2 mmol of triethylamine and mix well.
[0065] 4) Add 0.15 mmol of iminodiacetic acid and react overnight at room temperature.
[0066] 5) Evaporate the solvent under reduced pressure, dissolve the residue with 30 mL of dichloromethane, continue to add 30 mL of saturated saline for extraction, and separate the dichloromethane layer.
[0067] 6) Filter to remove impurities, use silica gel column chromatography, the eluent is petroleum ether:ethyl acetate=5:1, and obtain rose bengal powder with a total yield of 64%.
[0068] The synthetic product obtained above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com