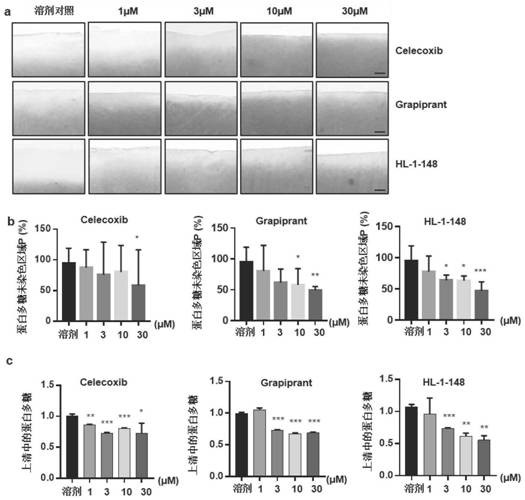
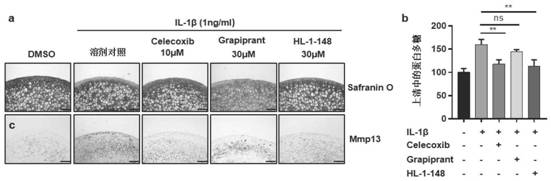
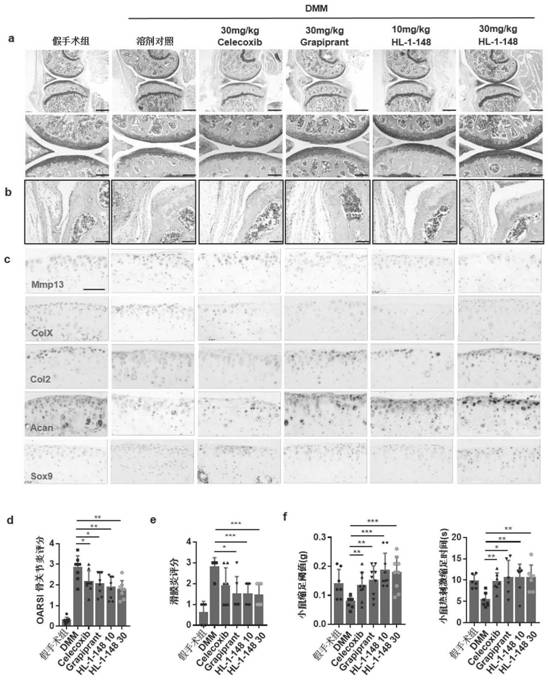
Targeted EP4 receptor micromolecule antagonist and application thereof in treatment of osteoarthritis and cartilage defects
A technology selected from and compounds, applied in the biological field, to achieve the effect of repairing articular cartilage damage, inhibiting catabolism, and reducing osteophyte formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0128] Example 1-1, Compound (S)-4-(1-(1-(2-(4-fluorophenoxy)ethyl)-4-phenyl-1hydrogen-1,2,3-triazole - Preparation of 5-formylamino) ethyl) benzoic acid
[0129]
[0130] Add 1-(2-bromoethoxy)-4-fluorobenzene (657mg, 3.00mmol) and sodium azide (215mg, 3.30mmol) to anhydrous dimethylsulfoxide (8ml), stir at room temperature for 12 hour, add water and ethyl acetate to extract after the reaction is over, take the organic layer and add a certain amount of anhydrous sodium sulfate, filter, and evaporate the organic solvent to dryness under reduced pressure without further purification to obtain the crude product 1-(2-azidoethyl Oxy)-4-fluorobenzene (516 mg, 95% yield).
[0131] 1-(2-azidoethoxy)-4-fluorobenzene (516mg, 2.85mmol) was added to the toluene solution (15ml), then ethyl phenylpropiolate (496mg, 2.85mmol) was added, at 120 Reflux at ℃ for 8 hours, cool to room temperature after the reaction, evaporate the organic solvent to dryness under reduced pressure, and purify...
Embodiment 1-2
[0135] Example 1-2, Compound 4-(1-1-(2-(4-fluorophenoxy)ethyl)-4-phenyl-1hydrogen-1,2,3-triazole-5-formyl Preparation of amino)cyclopropyl)benzoic acid
[0136]
[0137] Substitute (S)-methyl 4-(1-aminoethyl)benzoate with methyl 4-(1-aminocyclopropyl)-benzoate, and prepare according to the method of Example 1-1. 1 H NMR (500MHz, DMS0) δ12.86(s, 1H), 9.69(s, 1H), 7.81(d, J=8.5Hz, 2H), 7.66(d, J=8.2Hz, 2H), 7.45(dd J=15.6,8.2Hz,3H),7.36(d,J=8.5Hz,2H),7.09(t,J=8.8Hz,2H),6.92(dd,J=9.2,4.4Hz,2H),4.84( t, J=5.0Hz, 2H), 4.37(t, J=5.0Hz, 2H), 1.36(t, J=6.4Hz, 2H), 1.30(t, J=5.8Hz, 2H).
Embodiment 1-3
[0138] Example 1-3, Compound (S)-4-(1-(1-(2-(3-(trifluoromethyl)phenoxy)ethyl)-4-phenyl-1hydrogen-1,2 , the preparation of 3-triazole-5-carboxamido) ethyl) benzoic acid
[0139]
[0140] Replace 1-(2-bromoethoxy)-4-fluorobenzene with 1-(2-bromoethoxy)-3-(trifluoromethyl)benzene, and prepare according to the method of Example 1-1. 1 H NMR (500MHz, DMS0) δ12.80(s, 1H), 9.57(d, J=7.7Hz, 1H), 7.88(d, J=8.1Hz, 2H), 7.65(d, J=5.3Hz, 2H ), 7.48(t, J=9.8Hz, 3H), 7.39(d, J=2.4Hz, 3H), 7.29(d, J=7.5Hz, 1H), 7.17(d, J=7.1Hz, 2H) , 5.31-5.21 (m, 1H), 4.89-4.77 (m, 2H), 4.51-4.38 (m, 2H), 1.42 (d, J=6.9Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com