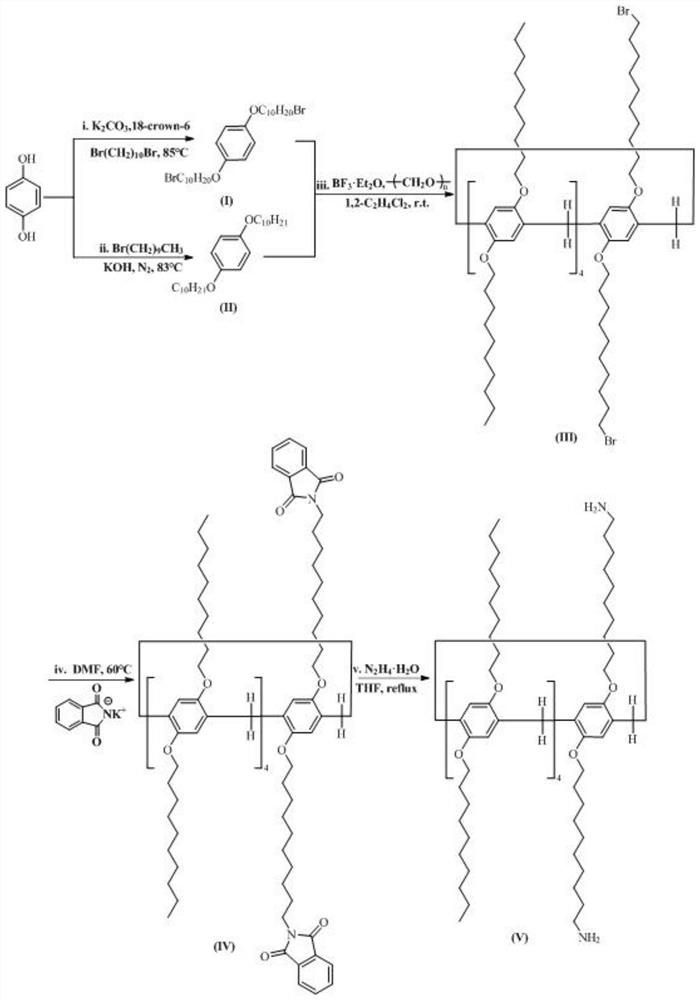
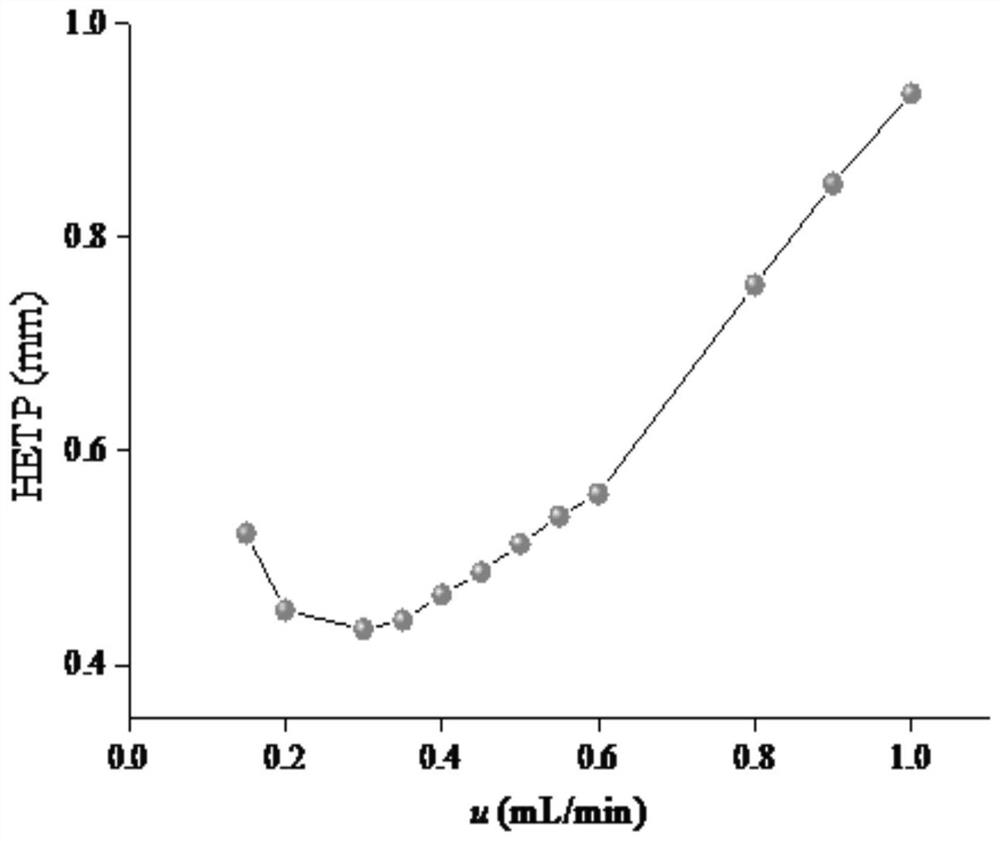
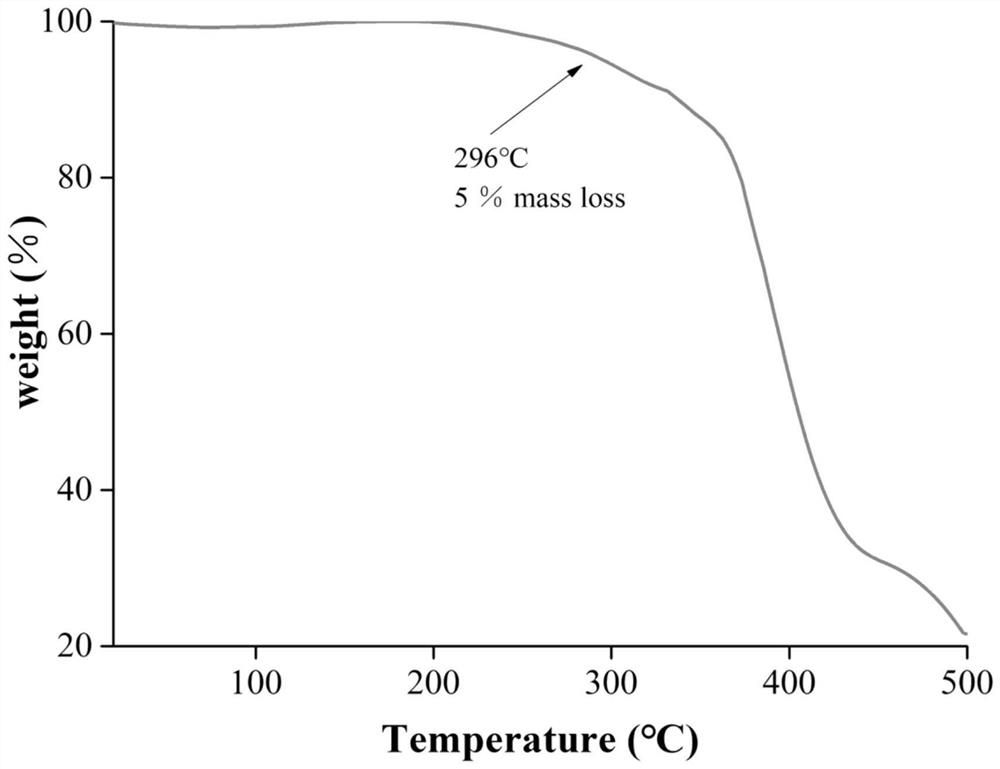
Preparation and application of amino-functionalized pillar penta-arene stationary phase
An amino-functionalized, column-pentarene technology is applied in the field of chromatographic analysis to achieve the effects of improving thermal stability and film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] 1.0g (9.08mmol) 1,4-hydroquinone, 11.70g (39.00mmol) 1,10-dibromodecane, 6.52g (47.18mmol) potassium carbonate and 0.04g (0.15mmol) 18-crown ether -6 was added to 40 mL of 2-butanone, heated to 85°C for 16 hours, cooled, filtered, and the filter cake was washed with 100 mL of dichloromethane. The filtrate was evaporated to dryness to obtain 11.20 g of a yellow viscous crude product, which was purified by column chromatography, and the eluent was petroleum ether:dichloromethane=5:1 (V:V), and intermediate (I) was obtained as a white solid: 1.91 g . m.p.89.9-90.6℃.IR(KBr,cm -1 ):643.84(C-Br), 1036.54(C-O-C), 1216.81(C-O-C), 1438.73(C=C), 1461.43(C=C), 1473.71(C=C), 1508.01(C=C), 2850.71(CH 2 ), 2918.83 (CH 2 ), 2933.37 (CH 3 ).
[0057]
[0058] Add 1.1g (10mmol) 1,4-hydroquinone, 6.85g (31mmol) 1-bromodecane, and 1.74g (31mmol) potassium hydroxide to 25mL ethanol, heat to 81°C for 10h, cool down, pump Filter, and wash the filter cake with deionized w...
Embodiment 2
[0066] The difference between this example and Example 1 is: the reaction conditions are different, and the purification method of step 4 is different
[0067] 2g (18.16mmol) 1,4-hydroquinone, 21.80g (72.65mmol) 1,10-dibromodecane, 12.56g (90.82mmol) potassium carbonate and 0.08g (0.30mmol) 18-crown ether- 6 was added to 70mL of 2-butanone, reacted at 85°C for 16h, cooled down, filtered, and the filter cake was washed with 100mL of dichloromethane. The filtrate was collected and evaporated to dryness to obtain 20.250 g of a yellow viscous crude product, which was purified by column chromatography, and the eluent was petroleum ether:dichloromethane=5:1 (V:V) to obtain intermediate (I): 4.04 g.
[0068] Add 2.2g (20mmol) 1,4-hydroquinone, 13.26g (60mmol) 1-bromodecane, 3.37g (60mmol) potassium hydroxide to 50mL ethanol, react at 85°C for 8h, cool down, and filter with suction , the filter cake was washed with deionized water until neutral. Recrystallize twice (2×150 mL) with d...
Embodiment 3
[0073] This example differs from Example 1 in that: the reaction conditions are different, and the purification method in step 4 is different.
[0074] 2g (18.16mmol) 1,4-hydroquinone, 21.80g (72.65mmol) 1,10-dibromodecane, 12.56g (90.82mmol) potassium carbonate and 0.08g (0.30mmol) 18-crown ether- 6 was added to 70 mL of 2-butanone, reacted at 83°C for 18 hours, cooled down, filtered, and the filter cake was washed with 100 mL of dichloromethane. The filtrate was collected and evaporated to dryness to obtain 20.801 g of a yellow viscous crude product, which was purified by column chromatography, and the eluent was petroleum ether:dichloromethane=5:1 (V:V) to obtain intermediate (I): 4.20 g.
[0075] Add 4.4g (40mmol) 1,4-hydroquinone, 26.52g (120mmol) 1-bromodecane, 6.73g (120mmol) potassium hydroxide to 100mL ethanol, react at 85°C for 8h, cool down, and filter with suction , the filter cake was washed with deionized water until neutral. Recrystallized twice (2×150 mL) wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com