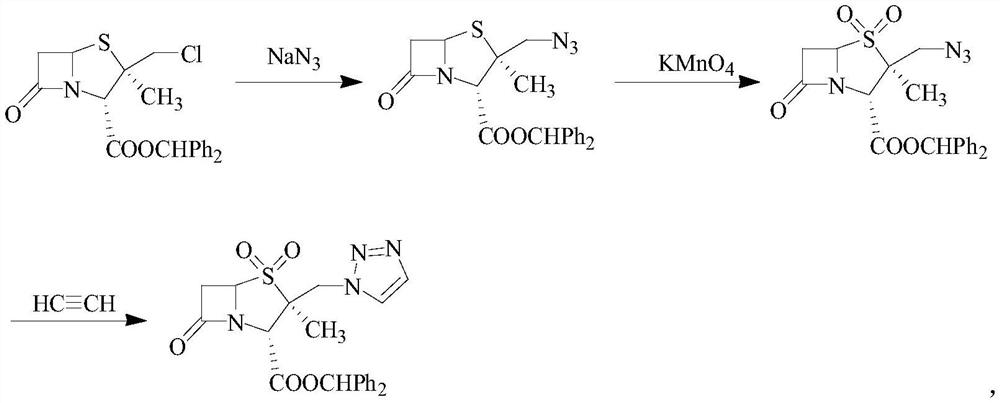
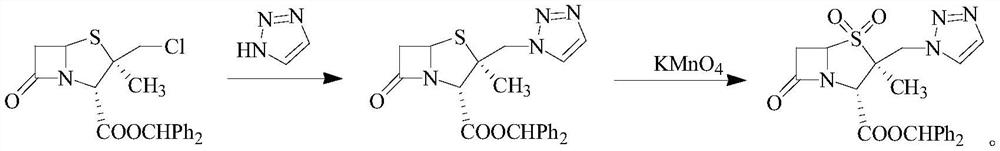
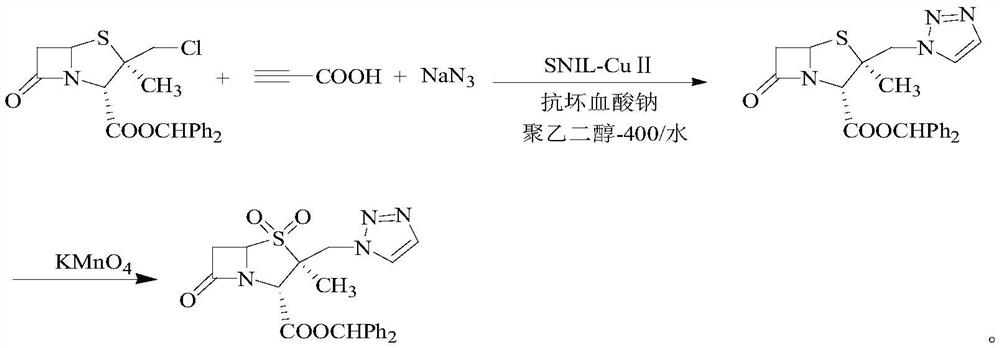
Synthetic method of tazobactam diphenylmethyl ester
A technology for the synthesis of zobactam diphenylmethyl ester and its synthesis method, which is applied in the field of synthesis of tazobactam diphenylmethyl ester, can solve the problems of unfavorable industrial production, large amount of triazole consumption, difficult recovery, etc., and achieve high recovery rate , reduce reaction time, shorten the effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] (1) Preparation of nano silicon dioxide
[0042] Prepare a mixture of n-pentanol (35ml) and sodium dodecylbenzenesulfonate (35g), add cyclohexane (165ml) and ultrasonically shake evenly, then transfer to water (10ml) and stir at room temperature for 30min, and finally add orthosilicic acid Ethyl esters continue to react. Centrifuge, wash with ethanol, and dry in vacuum at 100° C. for 2 hours to obtain nano-silica.
[0043] (2) Preparation of 1,2-bis(4-pyridinethio)ethane
[0044] 4-bromopyridine hydrochloride (1g), 1,2-ethanedithiol (0.3ml), sodium hydroxide (0.3g) were added to DMF (10ml) and reacted at 80°C for 24h; after the reaction, the 10ml of water and 10ml of ethyl acetate were added to the reaction solution and filtered, the filtrate was extracted and layered to obtain an aqueous phase and an organic phase, and then the aqueous phase was concentrated under reduced pressure, and finally the obtained concentrate was recrystallized with methanol. 1,2-Bis(4-pyri...
Embodiment 1
[0050] (1) Preparation of 2β-azidomethylpenicillanic acid diphenylmethyl ester
[0051] Add 0.01mol of nano-loaded Cu ionic liquid catalyst and 0.02mol of sodium ascorbate to polyethylene glycol-400 / water (1:1, 200mL) and stir for 15min, then add 42g (0.1mol) of 2β-chloromethyl Penicillium Diphenylmethyl alkanoate, 7.8g (0.12mol) NaN 3 and 7g (0.1mol) propiolic acid were added to the reaction system, stirred at room temperature for 2.5h, after the reaction was completed, 200mL ethyl acetate was added, centrifuged, the obtained catalyst was washed with acetone and water and dried for repeated use The organic layer was separated, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure, recrystallized from ethanol to obtain 40.9g of 2β-azidomethylpenicillanic acid diphenylmethyl ester, yield 90.4% [with 2β-chloro Diphenylmethyl penicillanic acid is calculated, yield=dry product weight / (2β-chloromethyl penicillanic acid diphenylmethyl ester mass×1...
Embodiment 2
[0055] (1) Preparation of 2β-azidomethylpenicillanic acid diphenylmethyl ester
[0056] Add 0.03 mol of nano-loaded Cu ionic liquid catalyst and 0.03 mol of sodium ascorbate into polyethylene glycol-400 / water (1.5:1, 200 mL) and stir for 20 min, then add 42 g (0.1 mol) of 2β-chloromethyl Penicillium Diphenylmethyl alkanoate, 8.5g (0.13mol) NaN 3 and 10.5g (0.15mol) propiolic acid were added to the reaction system, stirred at room temperature for 3h, after the reaction was completed, 200mL ethyl acetate was added, centrifuged, the obtained catalyst was washed with acetone and water and dried for repeated use The organic layer was separated, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure, recrystallized from ethanol to obtain 41.2g of 2β-azidomethylpenicillanic acid diphenylmethyl ester, yield 91.09% [with 2β-chloro Diphenylmethyl penicillanic acid is calculated, yield=dry product weight / (2β-chloromethyl penicillanic acid diphenylmethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com