Preparation method of integrated phosphine light emission carbazolyl organic photoelectric material
An organic photoelectric material, carbazole-based technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., to achieve low cost and environment-friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of 4,4'-difluoro-2,2'-dinitro-biphenyl:
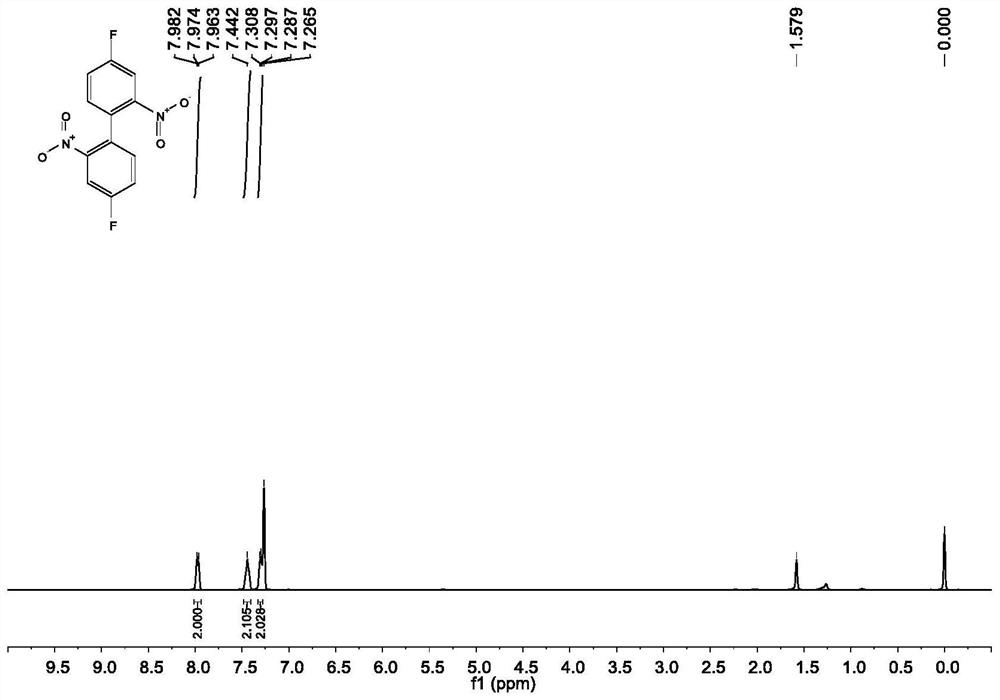
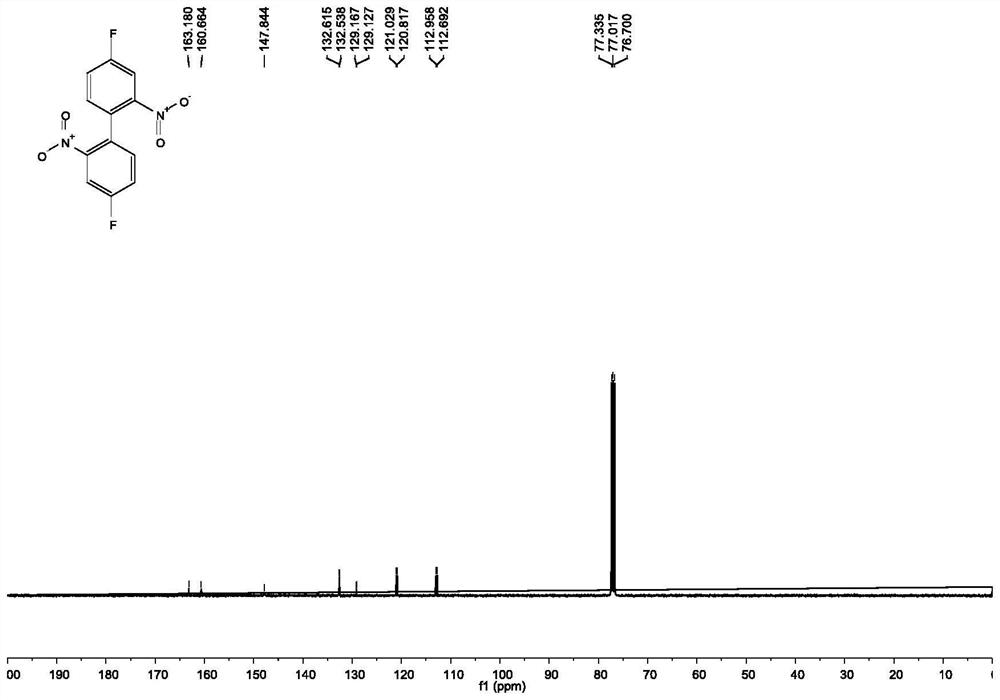
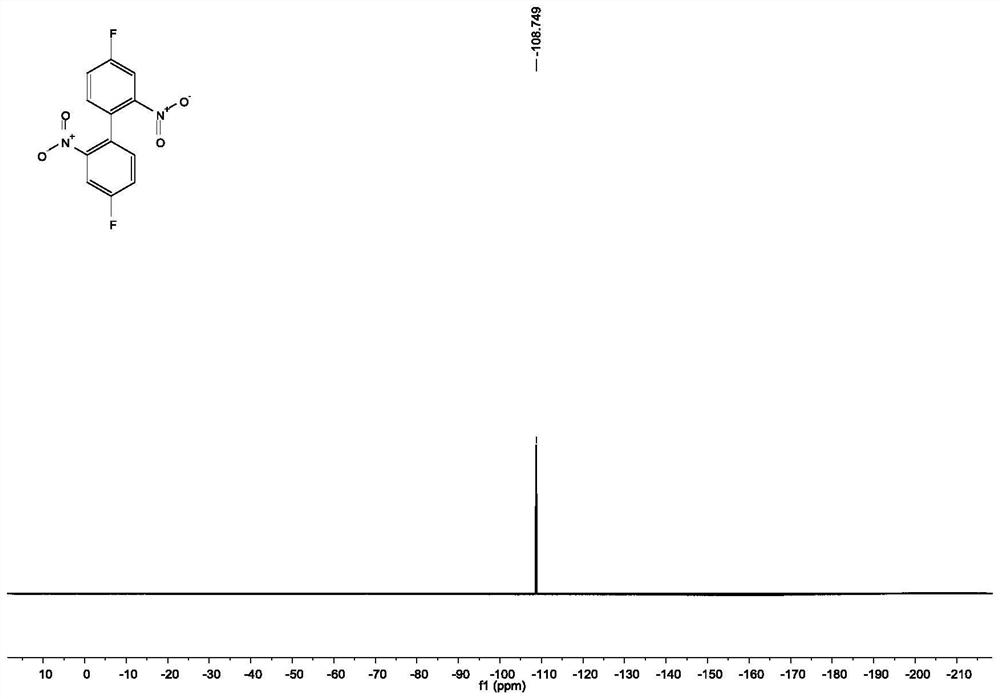
[0049] Under the protection of nitrogen, add 100mmol 2-bromo-5-fluoronitrobenzene, 220mmol copper powder and 200mL DMF to a 500mL thick-walled eggplant-shaped bottle equipped with a magnet, stir well at room temperature, and then maintain the reaction temperature at 125°C React for 4 hours. After the reaction of 2-bromo-5-fluoronitrobenzene is complete, cool to room temperature, filter, wash the filter cake with ethyl acetate, dilute the filtrate with ethyl acetate and wash with water and saturated brine in sequence. After washing, the organic phase is washed with Anhydrous Na 2 SO 4 Dry, filter, and spin dry to obtain 4,4'-difluoro-2,2'-dinitro-biphenyl 3 with a yield of 95%.
Embodiment 2
[0051] Synthesis of 4,4'-difluoro-2,2'-diamino-biphenyl:
[0052] Under the protection of nitrogen, 27.4mmol of 4,4'-difluoro-2,2'-dinitrobiphenyl was dissolved in 33mL of ethanol and 33mL of 8M HCl mixed solution, and then 6.0eq of stannous chloride dihydrate was added in batches; The mixture was warmed and stirred at reflux for 4 hours. After the reaction is complete, cool to room temperature and remove ethanol under reduced pressure, pour the residue into a large amount of water, adjust the pH to 8-10 with NaOH, extract three times with ethyl acetate, combine the organic phases and sequentially wash with sodium bicarbonate and saturated salt Wash with water, dry the organic phase with anhydrous sodium sulfate, filter, and remove the solvent under reduced pressure to obtain crude 4,4'-difluoro-2,2'-diamino-biphenyl. The ethyl acetate / petroleum ether system was recrystallized to obtain pure 4,4'-difluoro-2,2'-diamino-biphenyl 4 with a yield of 92%.
Embodiment 3
[0054] Synthesis of 4,4'-difluoro-2,2'-diiodo-biphenyl:
[0055] Dissolve 20mmol of 4,4'-difluoro-2,2'-diamino-biphenyl in 30mL of 8M hydrochloric acid under temperature control -10°C to 0°C, keep the temperature and add 60mmol NaNO dropwise to the system 2 Saturated solution, continued to stir at this temperature for 30 minutes, then lowered the reaction temperature to -15°C, then added 76 mmol KI aqueous solution dropwise to the system, and after the dropwise addition, the reaction system was slowly raised to room temperature and stirred overnight. Sodium thiosulfate was quenched, extracted three times with ethyl acetate, the organic phases were combined and washed with sodium bicarbonate and saturated brine successively, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure to obtain 4,4'- Crude difluoro-2,2'-diiodo-biphenyl 5. Ethyl acetate / petroleum ether system column chromatography (petroleum ether / ethyl acetate=300 / 1-50 / 1), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com