Samarium manganese mullite type nickel-based catalyst for hydrogen production by autothermal reforming of acetic acid
A nickel-based catalyst, autothermal reforming technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, hydrogen, etc., can solve the problem of reducing active site exposure, reducing catalyst activity, catalyst Problems such as agglomeration and sintering can achieve the effect of improving anti-coke ability, excellent thermal stability, and improving transfer ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Weigh 2.000g of γ-Al 2 o 3 Pour it into a beaker while weighing 0.866g of Ni(NO 3 ) 2 ·6H 2 O, add a certain amount of deionized water to dissolve, and then add dropwise to γ-Al 2 o 3 middle; placed at room temperature for 12h, dried, and then calcined in a tube furnace at 700°C for 4h to obtain the CDUT-NA catalyst, which formed a catalyst supported on γ-Al 2 o 3 The Ni-based catalyst on the catalyst; the weight percentage of the catalyst in terms of oxides consists of: nickel oxide (NiO) is 10.0%, γ-Al 2 o 3 was 90.0%.
[0025] The reactivity evaluation of autothermal reforming of acetic acid was carried out in a continuous flow fixed bed reactor. Grind and tablet the catalyst, then sieve it into 20-40 mesh particles, weigh 0.1-0.2g and put it into the reactor, and put it under H at a temperature of 500-800°C 2 The mixture solution of acetic acid and water was then injected into the vaporizer with a constant flow pump and vaporized, mixed with oxygen, and ni...
Embodiment 1
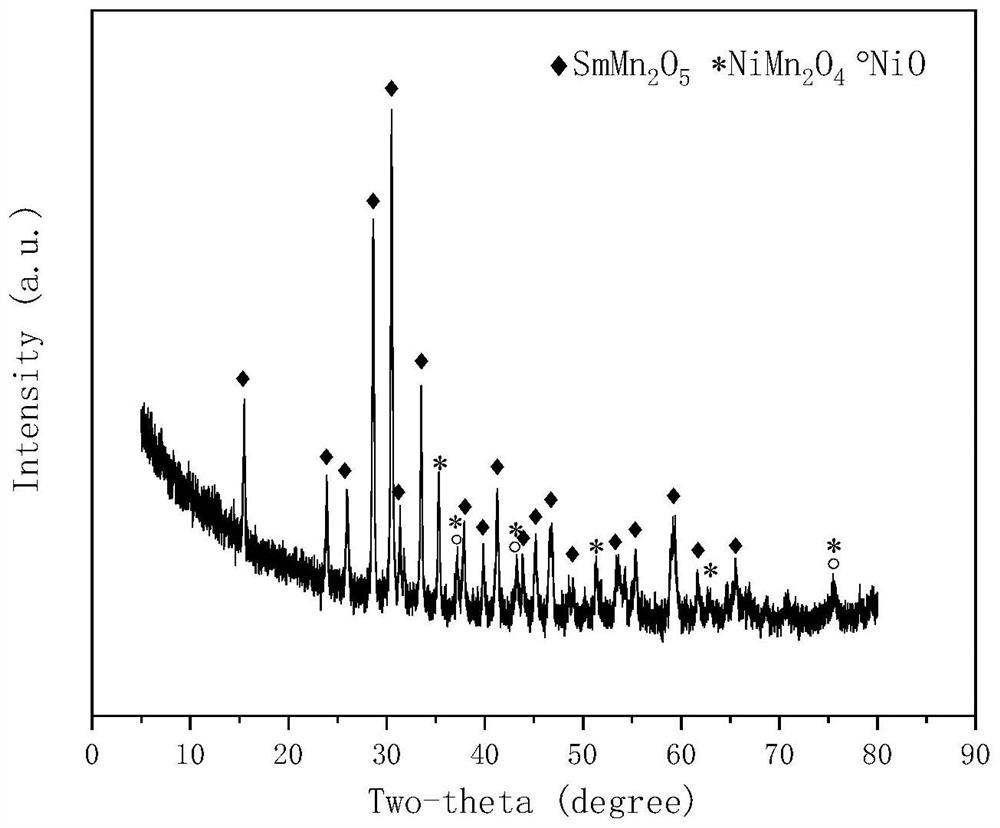
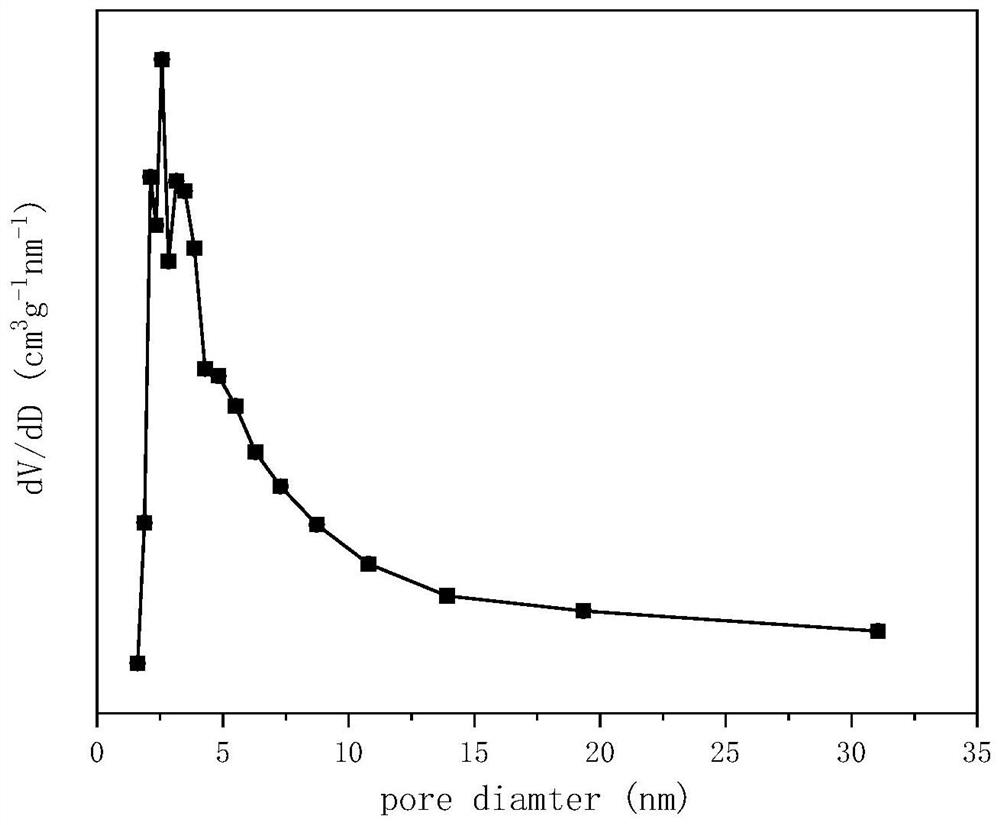
[0028] Weigh 7.203g of Sm(NO 3 ) 3 ·6H 2 O and 6.532g of Mn(CH 3 COO) 2 4H 2 O, dissolved in 30ml of ethylene glycol and 20ml of ethanol solution, stirred for 24h, placed in an oven at 80°C for 8h, then placed in a muffle furnace and roasted at 500°C for 8h, and finally roasted in a tube furnace at 800°C for 8h , to obtain samarium manganese mullite oxide SmMn 2 o 5 Carrier, add 20ml of water, stir to form a suspension; prepare a nickel nitrate solution, add it to the suspension of samarium manganese mullite, add ammonia water, adjust the pH value to 11±0.5, keep it in a water bath at 60°C for 3 hours, put it in 80 ℃ oven for 10 hours, and then baked in a tube furnace at 750 ℃ for 5 hours to obtain the CDUT-NSM-18 catalyst, forming a catalyst supported on SmMn 2 o 5 spinel NiMn on 2 o 4 Oxide and a small amount of NiO, after reduction, a mesoporous structure with Ni-Mn-Sm-O active centers is obtained, and the pore size is concentrated at 4nm. Its typical crystal st...
Embodiment 2
[0031] Weigh 7.203g of Sm(NO 3 ) 3 ·6H 2 O and 6.532g of Mn(CH 3 COO) 2 4H 2 O, dissolved in 30ml of ethylene glycol and 20ml of ethanol solution, stirred for 24h, placed in an oven at 80°C for 8h, then placed in a muffle furnace and roasted at 500°C for 8h, and finally roasted in a tube furnace at 800°C for 8h , to obtain samarium manganese mullite oxide SmMn 2 o 5 Carrier, add 20ml of water, stir to form a suspension; prepare a nickel nitrate solution, add it to the suspension of samarium manganese mullite, add ammonia water, adjust the pH value to 11±0.5, keep it in a water bath at 60°C for 3 hours, put it in 80 ℃ in an oven for 10 h, and then baked in a tube furnace at 750 °C for 5 h to obtain 2 o 5 spinel-containing NiMn 2 o 4 A CDUT-NSM-15 catalyst with a mesoporous structure of oxide and Ni-Mn-Sm-O active center; the catalyst is composed of 15.0% nickel oxide NiO, mullite oxide SmMn 2 o 5 was 85.0%.
[0032] Catalyst CDUT-NSM-15 was investigated by autother...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com