Transaminase and application thereof in preparation of optical pure chiral amine
A technology of transaminase and enzyme preparation, applied in the field of biochemistry, can solve the problems of low specificity of enzyme substrate, poor enantiomeric selectivity, low conversion rate, etc., and achieve high reaction efficiency, high enantiomeric selectivity and high conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0084] Using the sequence of SEQ ID NO: 1 (coding sequence as shown in SEQ ID NO: 3) as the parent, adopting strategies such as rolling PCR, iterative saturation mutation, and combined mutation to carry out directed evolution transformation, and then transform the mutant into Escherichia coli BL21 (DE3) competent cells were evenly spread on LB agar plates with 50 μg / ml kanamycin, and placed in a 37°C incubator for static culture for 18 hours. The mutants on the transformed plate were picked with a toothpick into a 96-well plate, and cultured overnight at 37° C. in a shaker at 220 rpm. Take 50 microliters of bacterial liquid from the hole of the first plate and insert it into the corresponding hole of the second plate, incubate at 37°C and 220rpm for 2-3h, add IPTG with a final concentration of 0.2mM, and incubate at 30°C for 20h to obtain the corresponding Mutants were subjected to high-throughput screening. Combined with SFC detection and re-screening, mutants with significa...
Embodiment 1
[0089] Prepare basic phosphate buffer: weigh 27.8g of dipotassium hydrogen phosphate trihydrate and 10.6g of potassium dihydrogen phosphate, add 200mL of purified water, stir at room temperature until the solid dissolves, adjust the pH to 6.9-7.1, add distilled water to make up to 2L.
[0090] Prepare basic isopropylamine buffer solution: add 280 ml basic phosphate buffer solution, 84 ml isopropylamine and 40 ml 85% phosphoric acid into a 500 ml glass bottle to obtain basic isopropylamine buffer solution.
[0091] Add 3 ml of basic isopropylamine buffer solution to the 8 ml reaction bottle, and adjust the pH between 8.8 and 9.1. Add 20 mg of transaminase (amino acid sequence as shown in SEQ ID NO:1) lyophilized powder and 2 mg of pyridoxal 5-phosphate (PLP) to the reaction solution, stir until the solid is completely dissolved, add 50 mg of substrate and 100 microliters Dimethyl sulfoxide was stirred at 28-32°C for 16 hours to fully react.
[0092] The conversion rate detecte...
Embodiment 2
[0096] Add 28 ml of basic phosphate buffer solution, 9.8 ml of sec-butylamine and 2.2 to 3.0 ml of 85% phosphoric acid in a 100 ml jacket to adjust the pH between 8.5 and 9.0. Add 400 mg of transaminase transaminase (amino acid sequence shown in SEQID NO: 2) freeze-dried powder and 20 mg of PLP to the reaction solution, stir until the solid is completely dissolved, add 1 gram of substrate, and react in a closed manner at 28 to 32°C for 64 hours. It's fully responsive.
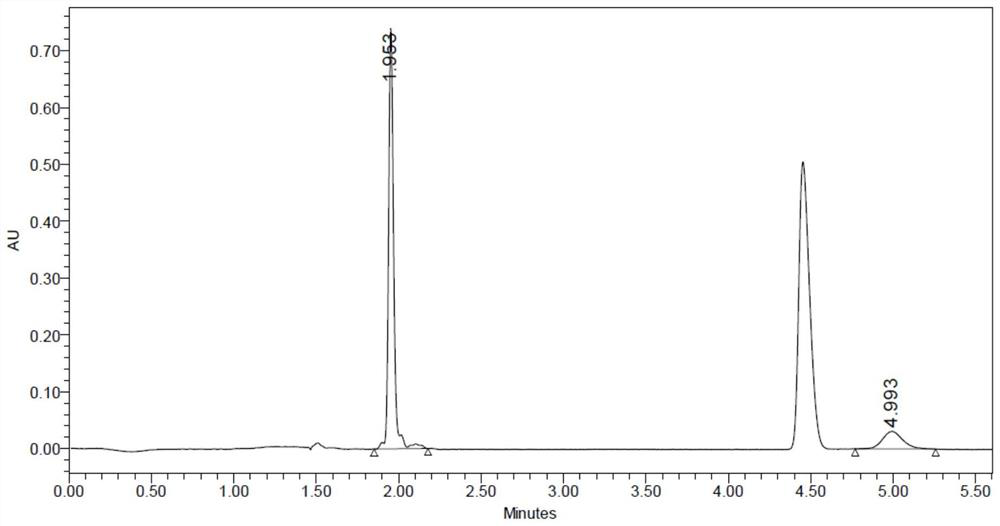
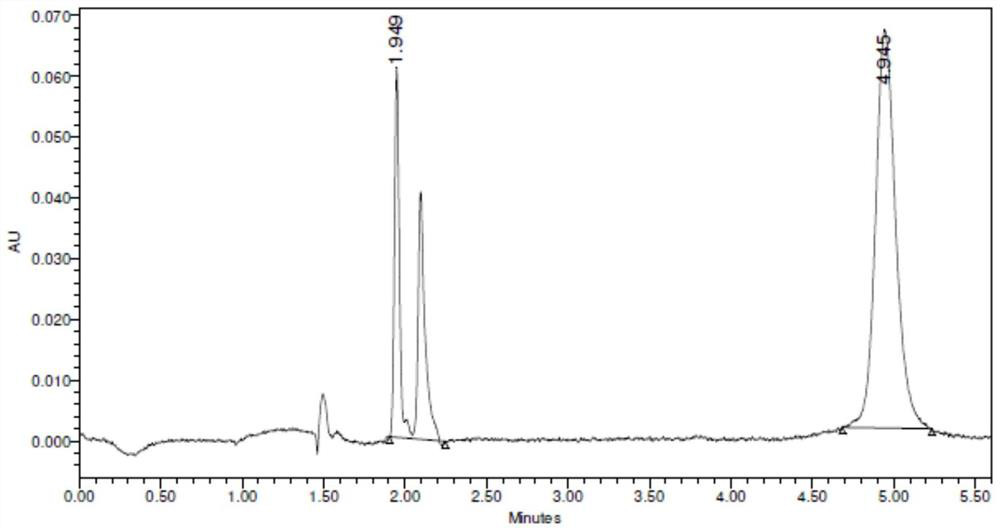
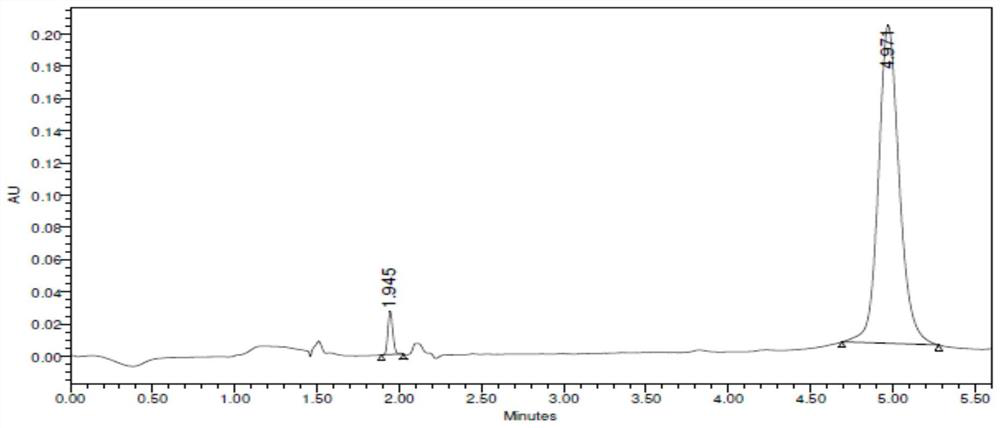
[0097] The conversion rate detected by SFC was 69.4%, and the ee value was >99%. Such as figure 2 Table 2 and Table 2 show that the peak at t=4.9min in the spectrum is the target compound (R)-1-(3-fluorophenyl)ethylamine.
[0098] Table 2
[0099]
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com