Efficient xyloglucanase and application thereof
A xyloglucanase and high-efficiency technology, applied in the field of xyloglucanase, can solve the problem of not finding xyloglucanase, etc., and achieve the effects of excellent enzyme activity, high yield and high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
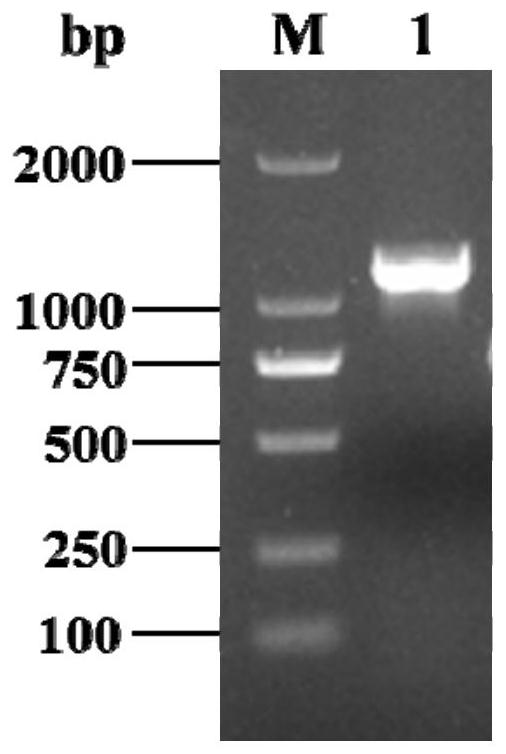
[0100] Enzyme specific activity definition: under the set assay conditions, the enzyme activity per mg of protein (U / mg). Example 1 Obtaining of CvXEG1 gene cDNA
[0101] 1. Extraction of grape white rot fungus RNA
[0102] Inoculate Ascospora viticola on PDA medium, culture for 2-3 days, gently scrape the mycelia with a scalpel, and crush the mycelium with liquid nitrogen grinding method. The mortar, pestle and tweezers used must be subjected to high temperature Sterilization. Use the column type fungal total RNA extraction and purification kit to extract grape white rot fungus RNA, and follow the product instructions.
[0103] 2. Reverse transcription and amplification of CvXEG1 gene cDNA
[0104] According to the genome sequence analysis of Coniella vitis, the coding region of CvXEG1 gene is a single exon. Design the following primers:
[0105] 5'-GCTGAAGCTTACGTACACCCCCCAACCCCAC-3'
[0106] 5'-GAATTAATTCGCGGCATGATGATGATGATGATGCTCGGAACGCTTGCG-3'
[0107] Grape white r...
Embodiment 2
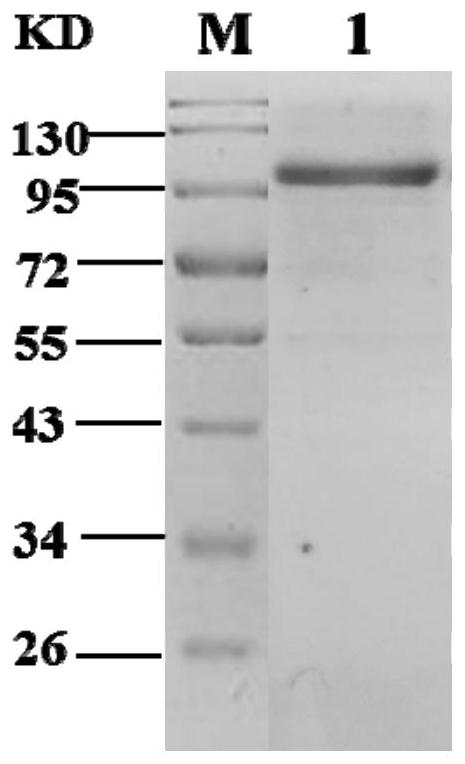
[0113] Example 2 Expression and purification of CvXEG1
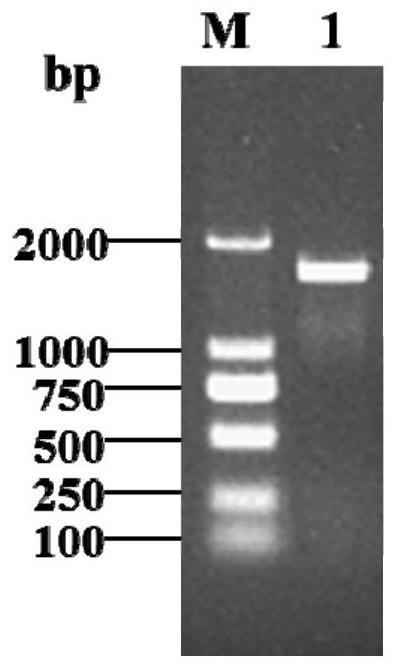
[0114] 1. Construction of pPIC9K-CvXEG1 recombinant expression vector
[0115] Plasmid pPIC9K was double-digested with restriction endonucleases EcoRI and NotI, and the double-digested product was recovered with a DNA purification and recovery kit. The CvXEG1 cDNA obtained in Example 1 was connected to the pPIC9K vector plasmid using a vector cloning kit and transformed into JM109 competent cells in Escherichia coli. After ampicillin antibiotic screening, PCR was performed on positive transformants using universal primers 5'AOX and 3'AOX, and positive transformants with correct band sizes were obtained. The transformant with the correct size of the verified band was sent to the company for sequencing, and the sequence of this piece of DNA was consistent with the sequence of SEQ ID 2 in the sequence listing.
[0116] 2 Construction of yeast strain containing pPIC9K-CvXEG1 recombinant plasmid
[0117] The pPIC9K-CvXEG1 ...
Embodiment 3
[0144] Example 3 Research on the enzymatic properties of CvXEG1
[0145] 1. Substrate-specific detection of recombinant proteins
[0146]Prepare 0.5% xyloglucan, 0.5% sodium carboxymethylcellulose, 0.5% xylan, 0.5% filter paper, and 0.5% microcrystalline cellulose respectively, and use The aforementioned methods measure the enzymatic activity of recombinant proteins on different substrates.
[0147] The results showed that the recombinant protein had high enzymatic activity on xyloglucan (specific activity up to 229U / mg), and at the same time, the enzyme also had certain enzymatic activity on xylan (specific activity up to 105U / mg). The results are shown in Table 3 below:
[0148] Table 3 Substrate-specific detection of recombinant protein pPIC9K-CvXEG1
[0149]
[0150] 2. Optimum working temperature
[0151] Under the condition of pH 5.0, a 0.2% xyloglucan solution was prepared, and the enzyme activity of the recombinant protein was measured at 30°C, 40°C, 50°C, 60°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com