Chiral diphosphine ligand, rhodium complex of chiral diphosphine ligand, preparation method and application of chiral diphosphine ligand and rhodium complex
A technology of chiral bisphosphine ligands and rhodium complexes, which is applied in the preparation of organic compounds, rhodium organic compounds, chemical instruments and methods, etc. It can solve the problems of low catalyst efficiency, unsuitable solvents, complex ligand structures, etc. problems, to achieve good application prospects, simple preparation process, and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
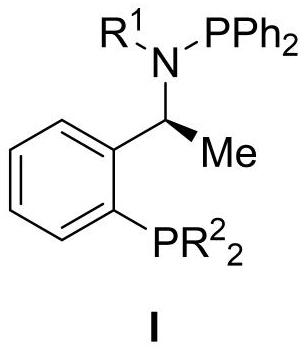
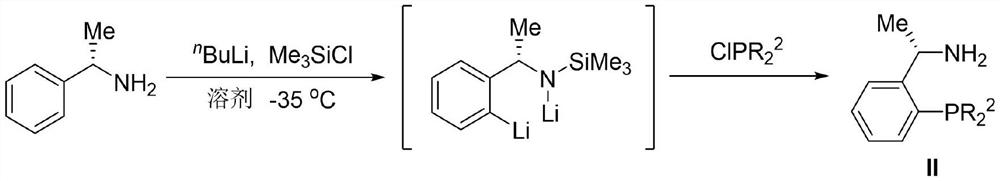
[0064] Embodiment 1: Preparation of intermediate (S)-1-(2-diarylphosphine) phenylethylamine IIa-IIc
[0065] (S)-1-(2-bis(3,5-dimethylphenyl)phosphine)phenylethylamine (IIa)
[0066]
[0067] A 250mL three-necked flask was placed under argon protection, and then ultra-dry diethyl ether (50mL) and (S)-1-phenylethylamine (3.82mL, 30mmol) were added, the system was stirred evenly and pre-cooled at -35°C. Under stirring condition, n-butyllithium (12mL, 30mmol, 2.5M in THF) was added dropwise to the system. After the dropwise addition was completed, stir at -35°C for 30min. Then, under stirring conditions at -35°C, trimethylchlorosilane (4.26mL, 30mmol) was added dropwise to the system. After the addition was completed, stir at -35°C for 1.5h. Add n-butyllithium (36mL, 90mmol, 2.5M in THF) dropwise to the reaction system. After the addition is complete, it rises to room temperature within 2h and stirs overnight. Afterwards, di( 3,5-Dimethylphenyl) phosphorus chloride in ether...
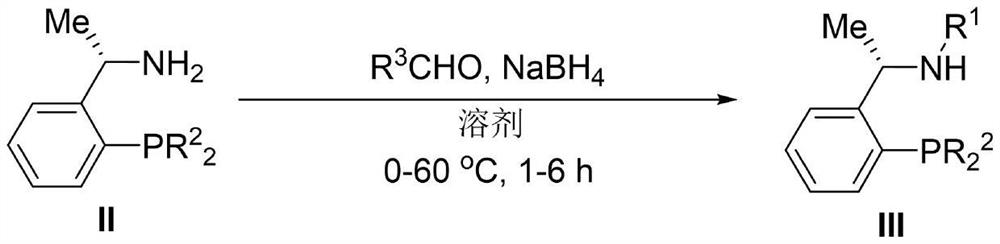
Embodiment 2
[0087]Example 2: Preparation of intermediate (S)-N-alkyl-1-(2-diarylphosphine)phenylethylamine IIIa-IIIe (S)-N-ethyl-1-(2-diphenyl Phosphine) phenylethylamine (IIIa)
[0088]
[0089] Add (S)-1-(2-diphenylphosphine) phenylethylamine (3.05g, 10mmol) and acetaldehyde (0.66g, 15mmol) in the 50mL Schlenck bottle to place the system under argon protection, then add super Dry methanol (20 mL), stir at room temperature for 1 h. Afterwards, the system was pre-cooled in an ice-water bath, and sodium borohydride (1.14 g, 30 mmol) was added quickly, the ice-water bath was removed, and the reaction was carried out at room temperature for 3 h. TLC monitors the reaction. After the reaction is over, add water to quench the system at 0°C under stirring conditions, add dichloromethane to the system until the two phases are clear, separate the liquid with a separatory funnel, and extract the aqueous phase with dichloromethane (50mL×3) , the organic phases were combined, washed with saturat...
Embodiment 3
[0123] Example 3: Preparation of chiral bisphosphine ligands Ia-Ie
[0124] Chiral bisphosphine ligand Ia
[0125]
[0126] Add IIIa (1.56g, 4.6mmol), steamed toluene (30mL) and steamed triethylamine (3.2mL, 23mmol) successively into a 100mL three-necked flask, freeze and degas the system, and place under argon protection. The system was pre-cooled in an ice-water bath, and then diphenylphosphine (1.3 mL, 6.9 mmol) was added dropwise. After the drop was complete, the ice-water bath was removed, and the reaction was carried out at 120° C. for 24 h. After TLC determined that the reaction was complete, the heating was stopped. After the system returned to room temperature, it was filtered through diatomaceous earth. =50:1, v / v), finally obtained chiral bisphosphine ligand Ia 1.72g, as a white solid, yield: 79%, melting point: 156-158°C (decomposition), [α] D 27 = 49.5 (c 0.50, CHCl 3 ).
[0127] 1 H NMR (400MHz, CDCl 3 )δ7.73(s,1H),7.51–7.20(m,21H),7.12(t,J=7.1Hz,1H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com