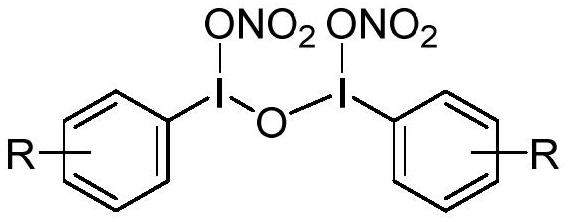
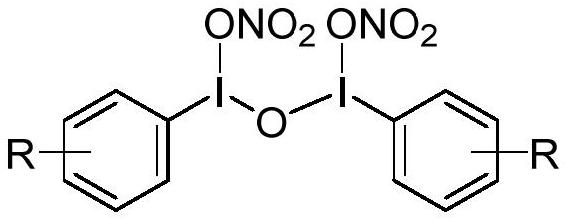
Nitrate group transfer reagent as well as preparation method and application thereof
A technique of nitrate and radical transfer, applied in the field of nitrate-based transfer reagent compound and its synthesis, novel aryl acyclic trivalent iodine nitrate-based transfer reagent and preparation thereof, can solve the complicated post-processing process and the application of substrates narrow range, poor atom economy, etc., to avoid column chromatography, significant social and economic benefits, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
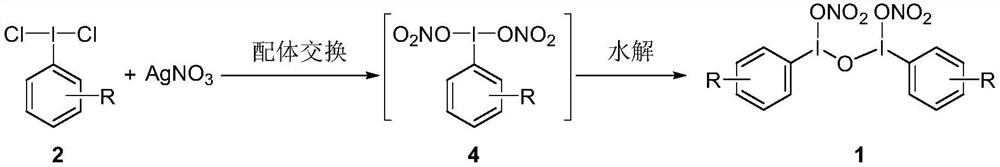
[0033] The preparation of phenyl acyclic trivalent iodine nitrate ester transfer reagent reacts according to the following reaction equation:
[0034]
[0035] The specific operation steps are:
[0036] 1) Dissolve compound 3a (iodobenzene, 12.2 g, 0.06 mol) in 200 ml of acetonitrile at room temperature, and then add sodium hypochlorite (10 wt%, 120 ml), water (200 ml) and analytically pure Concentrated hydrochloric acid (120 ml), stirred and reacted for 1 hour, filtered, washed with water, and dried to dichloroiodobenzene 2a (14.7 g, 89% yield);
[0037] 2) Dissolve compound 2a (dichloroiodobenzene, 13.8 g, 0.05 mol) in 300 ml of dichloromethane at room temperature in a nitrogen atmosphere, then add silver nitrate (25.5 g, 0.15 mol), stir overnight, and filter Silver chloride was removed, concentrated to obtain a yellow oil, dissolved in 50 milliliters of dichloromethane, then added an appropriate amount of water (0.5 liters per mole) and stirred for 30 minutes to obtain ...
Embodiment 2
[0041] The preparation of p-fluorophenyl acyclic trivalent iodine nitrate ester transfer reagent reacts according to the following reaction equation:
[0042]
[0043] The specific operation steps are:
[0044] 1) At room temperature, dissolve compound 3b (0.005 moles) in 20 milliliters of acetonitrile, and then add sodium hypochlorite (10 wt%, 10 milliliters), water (20 milliliters) and analytically pure concentrated hydrochloric acid (10 milliliters) in succession under stirring , stirred and reacted for 1 hour, filtered, washed with water, and dried to dichloroiodobenzene 2b (71% yield);
[0045] 2) Dissolve compound 2b (0.003 mol) in 20 ml of dichloromethane at room temperature in a nitrogen atmosphere, then add silver nitrate (0.09 mol), stir overnight, remove silver chloride by filtration, and concentrate to obtain a yellow oil. After being dissolved in 10 milliliters of dichloromethane, add a drop of water and stir for 10 minutes to obtain the nitrate group transfer...
Embodiment 3
[0049] The preparation of m-chlorophenyl acyclic trivalent iodine nitrate transfer reagent reacts according to the following reaction equation:
[0050]
[0051] The specific operation steps are:
[0052] 1) Dissolve compound 3c (0.005 mole) in 20 ml of acetonitrile at room temperature, and then add sodium hypochlorite (10 wt%, 10 ml), water (20 ml) and analytically pure concentrated hydrochloric acid (10 ml) in succession under stirring , stirred and reacted for 1 hour, filtered, washed with water, and dried to dichloroiodobenzene 2c (60% yield);
[0053] 2) Dissolve compound 2c (0.003 mol) in 20 ml of dichloromethane at room temperature in a nitrogen atmosphere, then add silver nitrate (0.09 mol), stir overnight, remove silver chloride by filtration, and concentrate to obtain a yellow oil. After being dissolved in 10 milliliters of dichloromethane, add a drop of water and stir for 10 minutes to obtain the nitrate group transfer reagent compound 1c (86% yield), a yellow s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com