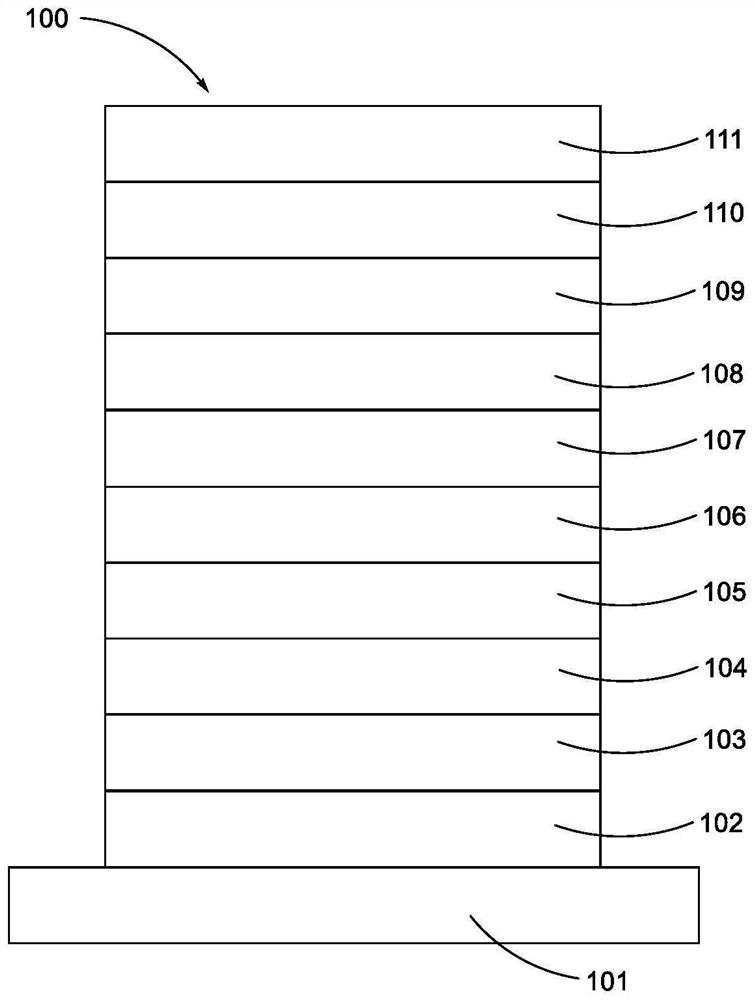
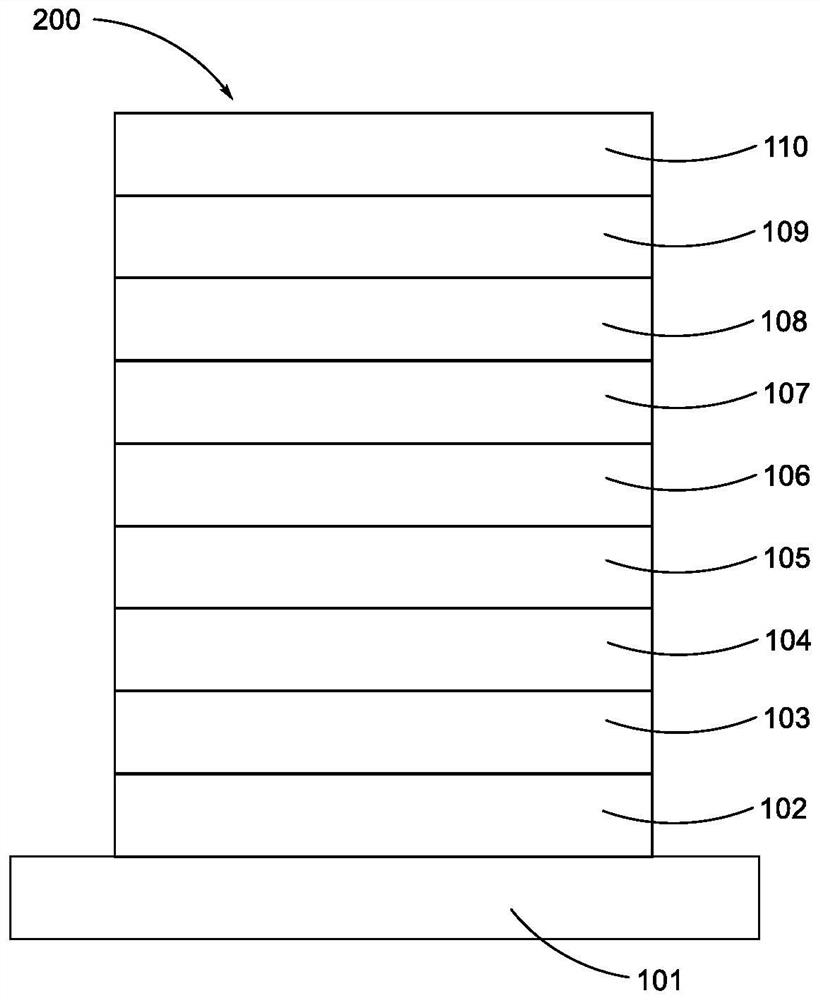
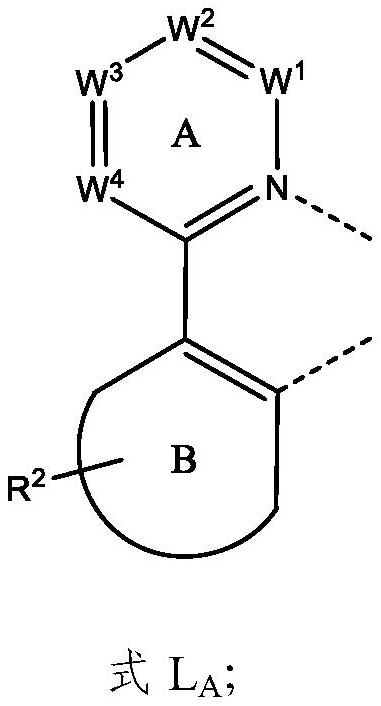
Metal complex and organic electroluminescent device
A technology of metal complexes and metals, applied in the fields of electric solid-state devices, organic chemistry, luminescent materials, etc., can solve the problems of reducing the quantum yield of the red light system, low luminous quantum efficiency, and aggravating phosphor quenching, achieving easy sublimation Purification, high luminous efficiency, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Preparation of Compound LA6:
[0094] The preparation method of ligand LA6 specifically comprises the following steps:
[0095] The first step: preparation of compound Int-1
[0096]
[0097] 20.0mmol of 3-iodo-2-cyanobenzothiophene (CAS: 2713542-25-5) and 20.0mmol of o-methoxyphenylacetylene, 2.0mmol of cuprous iodide, and then add 0.2mmol of PdCl 2 (PPh 3 ) 2 Catalyst and 60mL of triethylamine were reacted under nitrogen protection at room temperature for 12 hours, filtered, the filtrate was concentrated to dryness under reduced pressure, separated and purified by silica gel column to obtain compound Int-1, yellow solid, yield: 98%.
[0098] The second step: the preparation of compound Int-2
[0099]
[0100] 10.0mmol of the intermediate Int-1 prepared in the first step was dissolved in 50mL of dichloromethane, 10.0mmol of iodine was added, under the protection of nitrogen, the reaction was stirred at room temperature for 16 hours, and 50mL of saturated aque...
Embodiment 2
[0105] The preparation of compounds LA1~LA5, LA7~LA224, referring to the preparation method of Example 1, replaced 3-iodo-2-cyanobenzofuran or 3-cyano-2-iodobenzothiophene with different substituents For the 3-iodo-2-cyanobenzothiophene in the first step in Example 1, the o-ethynylanisole sulfide with different substituents is replaced with the o-methoxyphenylacetylene in the first step in Example 1, Replace the 3,5-dimethylphenylmagnesium bromide in the third step in Example 1 with phenylmagnesium bromide with different substituents, and adapt other test parameters to prepare LA1 in the ligand LA ~LA5, LA7~LA224.
Embodiment 3
[0107] Compound Ir(LA6) 2 Preparation of (LC4):
[0108] Metal complex Ir(LA6) 2 (LC4) preparation method, comprises the steps:
[0109] The first step: preparation of compound Int-3
[0110]
[0111] 10.0 mmol of compound LA6 and 5.0 mmol of IrCl 3 ·3H 2 O was dispersed in 30ml of ethylene glycol ether and 10ml of water, under the protection of nitrogen, the temperature was raised to reflux for 24 hours, cooled to room temperature, filtered, the filter cake was washed with water, and dried in vacuo to obtain compound Int-3, dark brown solid, yield : 83%.
[0112] The second step: Compound Ir(LA6) 2 Preparation of (LC4)
[0113]
[0114] 5.0mmol of compound Int-3 and 15.0mmol of 3,7-diethylnonane-4,6-dione and 25.0mmol of anhydrous potassium carbonate were dispersed in 40mL of acetonitrile and 40mL of chloroform, under nitrogen protection , heated to reflux for 24 hours, cooled to room temperature, poured the reaction solution into water, extracted with dichlorom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com