Palmatine hydrochloride-aspirin supramolecular compound
A supramolecular compound, palmatine hydrochloride technology, applied in the field of drug crystallization, can solve the problem that there is no public report on the preparation method and application of palmatine hydrochloride and aspirin supramolecular compound, and achieves green and efficient preparation method and crystal structure. Clear, physicochemically stable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Put 0.5 mmol of palmatine hydrochloride hydrate and 0.5 mmol of aspirin into 50 mL of absolute ethanol, heat and stir to obtain a clear mixed solution; dry the mixed solution at room temperature to obtain palmatine hydrochloride-aspirin supramolecular compound .
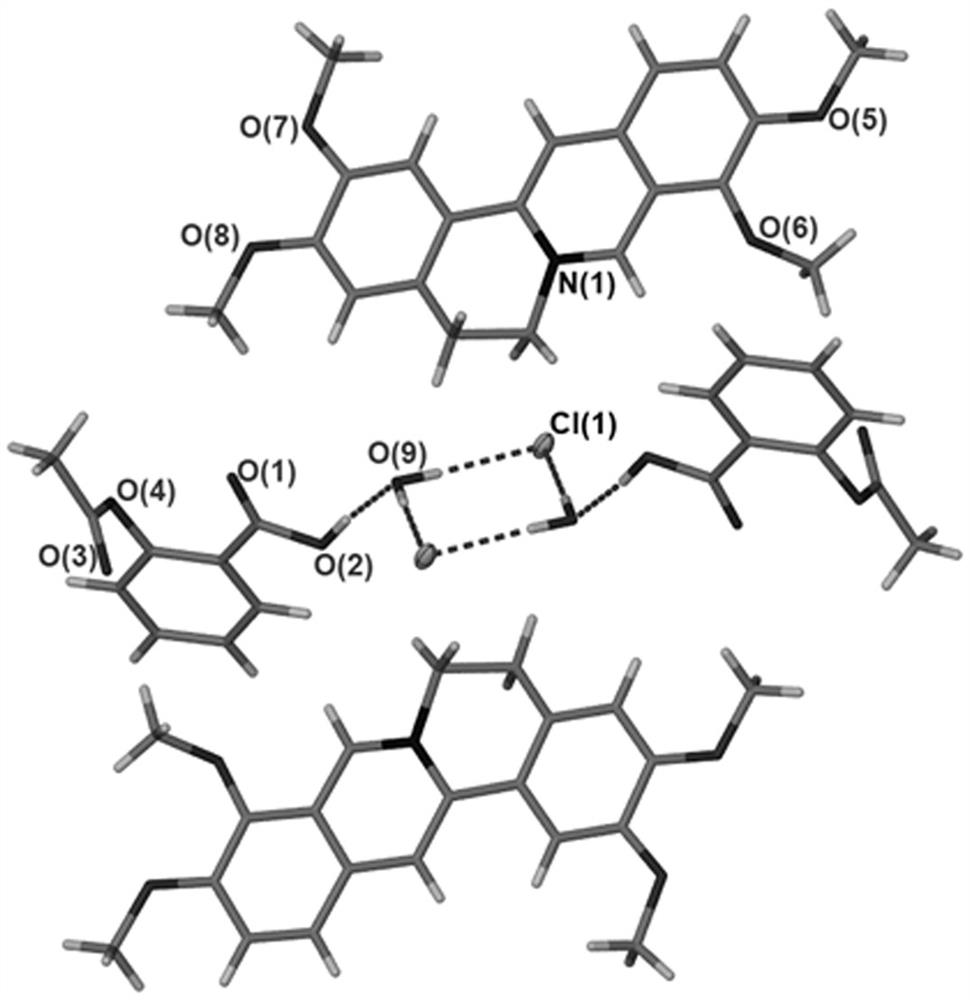
[0027] The palmatine hydrochloride-aspirin supramolecular compound prepared in this embodiment is characterized by single crystal X-ray diffraction, and the result shows that the structural unit of the supramolecular compound comprises palmatine hydrochloride molecule, aspirin molecule and water molecule, three The molar ratio between them is 1:1:1; the supramolecular compound belongs to the triclinic crystal system, the P-1 space group, and the unit cell parameters are: a =7.8368(7) Å, b = 11.5369(6) Å, c = 16.4916(8) Å, α = 76.151(4) º , β = 83.683(5) º , gamma = 73.338(6) º , V = 1385.54(17) Å 3 , Z = 2, D c = 1.405 g / cm 3 , the molecular formula is [C 21 h 22 ClNO 4 ]·[C 9 h 8 o 4 ] ...
Embodiment 2
[0031] Put 1 mmol of palmatine hydrochloride hydrate, 1 mmol of aspirin and 5 mL of absolute ethanol into a glass bottle with a lid and mix them, put them into a magnetic rotor and seal them, and stir magnetically at room temperature to form a suspension, and keep it for 24 hours. The resulting precipitate was filtered, washed with a small amount of absolute ethanol, and dried to obtain the palmatine hydrochloride-aspirin supramolecular compound.
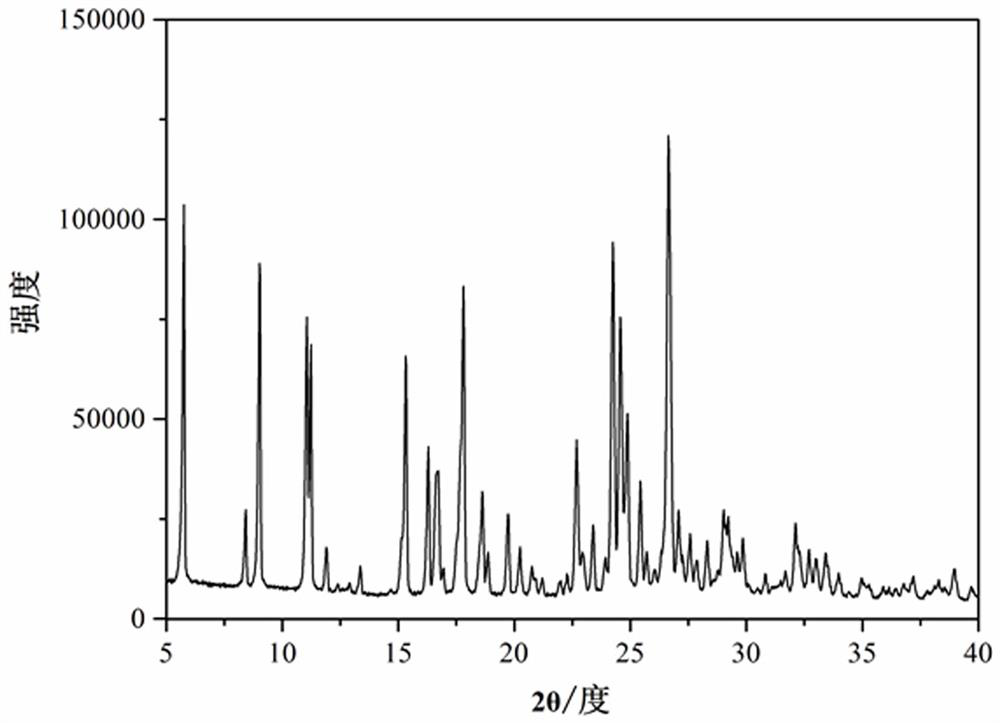
[0032] image 3 It is the powder X-ray diffraction pattern of the palmatine hydrochloride-aspirin supramolecular compound prepared in the present embodiment. Depend on image 3 Know, the palmatine hydrochloride-aspirin supramolecular compound that the present embodiment makes is identical with embodiment 1 θ The peaks appear at different angles, indicating that the two have the same crystal structure.
[0033] Figure 4 It is the dynamic water vapor adsorption diagram of palmatine hydrochloride hydrate and the palmatine hydroch...
Embodiment 3
[0036] Put 1 mmol of palmatine hydrochloride hydrate, 1 mmol of aspirin and 5 mL of deionized water into a glass bottle with a lid and mix them, put them into a magnetic rotor and seal them, and stir magnetically at room temperature to form a suspension, and keep it for 24 hours. The resulting precipitate was filtered, washed with a small amount of deionized water, and dried to obtain the palmatine hydrochloride-aspirin supramolecular compound.
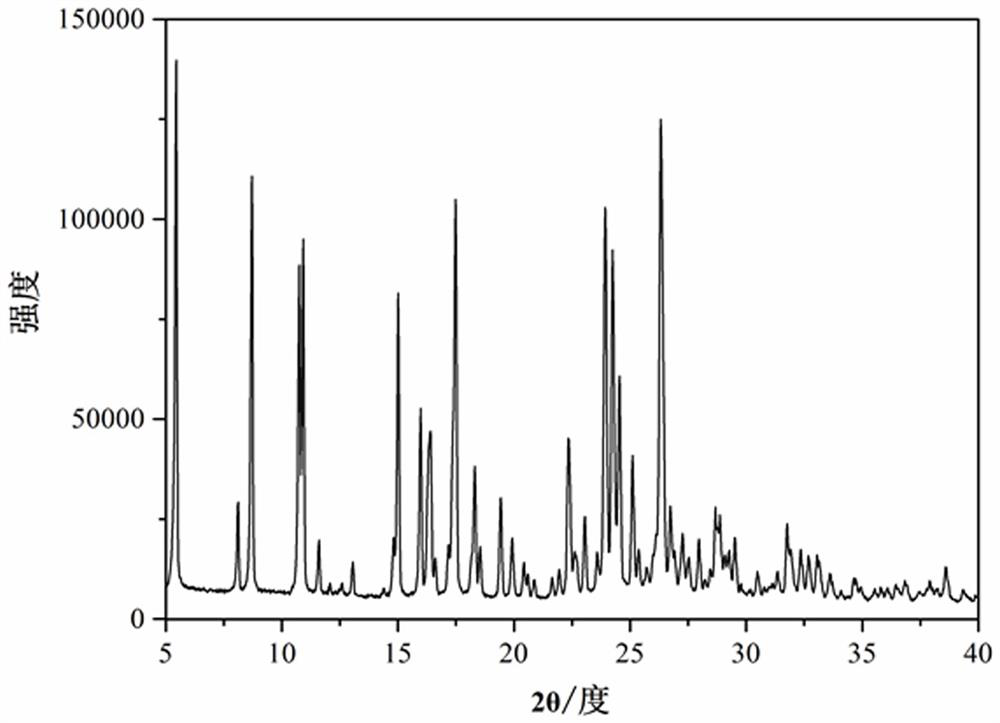
[0037] X-ray powder diffraction characterization result shows, the palmatine hydrochloride-aspirin supramolecular compound that the present embodiment makes is identical with embodiment 1. θ The peaks appear at different angles, indicating that the two have the same crystal structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com