Allylated monophosphine ligand and preparation method thereof
A technology of allylation mono- and allylation, which is applied in the field of new allylation monophosphine ligands and its preparation, can solve the problems of limiting biphenyl monophosphine ligands, and achieve low environmental pollution impact, Synthetic process is efficient and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
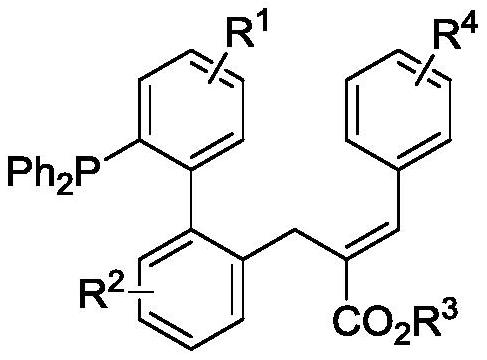
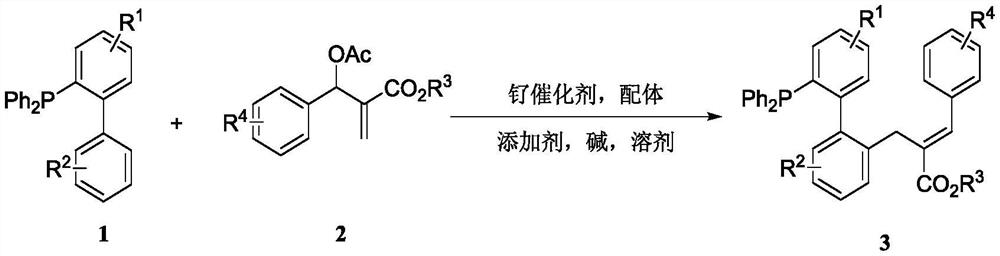
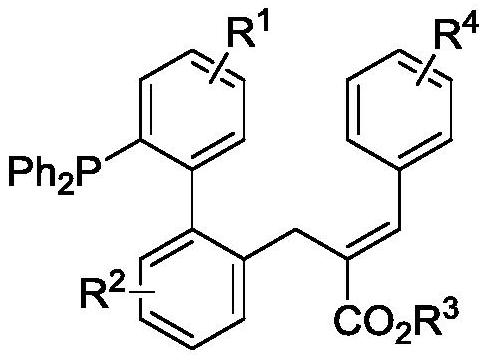
[0026] The novel allylated monophosphine ligand {(E)-2-((2'-(diphenylphosphino)-[1,1'-biphenyl]-2-yl)methyl)- 3-ethyl phenylacrylate}, the synthetic route is as follows:
[0027]
[0028] Add 33.8 mg (0.1 mmol) of 2-diphenylphosphine-biphenyl 1a, 49.6 mg of ethyl 2-(acetoxyphenylmethyl)acrylate 2a (0.2 mmol), 3.1 mg of p-cymene to the reaction kettle Ruthenium dichloride dimer, 7.3mg 1-tert-butoxycarbonamide cyclohexanecarboxylic acid, 1.8mg tris(4-methoxyphenyl) phosphine, 32.8mg sodium phosphate and 1.5mL n-hexane, after mixing evenly Pass argon into the reaction system, control the reaction in an argon atmosphere, and react at 120°C for 12 hours, then cool to room temperature, filter with diatomaceous earth, and distill under reduced pressure to obtain 39.5 mg of product 3a by column chromatography. rate of 75%. 1 H NMR (400MHz, CDCl 3 )δ7.96(s,1H),7.43-7.36(m,3H),7.35-7.26(m,7H),7.26-7.16(m,8H),7.10-6.97(m,4H),6.72(d, J=8.0Hz, 1H), 4.25-4.18(m, 2H), 3.80(q, J=32.0Hz...
Embodiment 2
[0036] In this example, the novel alkylated monophosphine ligand {3-(2'-(diphenylphosphino)-[1,1'-biphenyl]-2-yl) tert-butyl propionate}, synthetic route as follows:
[0037]
[0038] Added 37.2 mg (0.1 mmol) 4-chloro-2-diphenylphosphine-biphenyl 1b, 49.6 mg ethyl 2-(acetoxyphenylmethyl)acrylate 2a (0.2 mmol), 3.1 mg to the kettle p-cymene dichloride ruthenium dimer, 7.3 mg 1-tert-butoxycarbonamide cyclohexanecarboxylic acid, 1.8 mg tris(4-methoxyphenyl) phosphine, 32.8 mg sodium phosphate and 1.5 mL n-hexane, stirred After mixing evenly, pass argon into the reaction system, control the reaction in an argon atmosphere, and react at a temperature of 120°C for 12 hours, then cool to room temperature, filter with diatomaceous earth, and separate by column chromatography after vacuum distillation to obtain 50.4 mg of the product 3b, yield 90%. 1 H NMR (400MHz, CDCl3) δ7.96(s, 1H), 7.40-7.27(m, 9H), 7.26-7.13(m, 8H), 7.09-6.97(m, 3H), 6.93(dd, J=8.0 ,4.0Hz,1H),6.59(d,J=12.0Hz...
Embodiment 3
[0040] The novel alkylated monophosphine ligand (E)-2-((2'-(diphenylphosphino)-4'-methyl-[1,1'-biphenyl]-2-yl) in this example Methyl)-3-ethyl phenylacrylate, the synthetic route is as follows:
[0041]
[0042] Add 35.2 mg (0.1 mmol) (4-methyl-[1,1'-biphenyl]-2-yl) diphenylphosphine 1c, 49.6 mg 2-(acetoxyphenylmethyl) to the reaction kettle Ethyl acrylate 2a (0.2 mmol), 3.1 mg p-cymene dichloride ruthenium dimer, 7.3 mg 1-tert-butoxycarbonamide cyclohexanecarboxylic acid, 1.8 mg tris(4-methoxyphenyl)phosphine , 32.8mg of sodium phosphate and 1.5mL of n-hexane, stirred and mixed evenly, then passed argon into the reaction system, controlled the reaction in an argon atmosphere, and the temperature was 120°C for 12 hours, then cooled to room temperature, filtered with diatomaceous earth, After distillation under reduced pressure, 14.1 mg of product 3c was obtained by column chromatography, with a yield of 26%. 1 HNMR (400MHz, CDCl 3 )δ7.94(s,1H),7.40-7.36(m,2H),7.33-7.16(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com