Preparation method of alpha-alkenyl-alpha, alpha-difluoroaryl ketone compound and product
A technology for difluoroaryl ketones and compounds is applied in the field of organic compounds, can solve problems such as undeveloped ones, and achieve the effects of cheap catalyst, high economic benefit and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
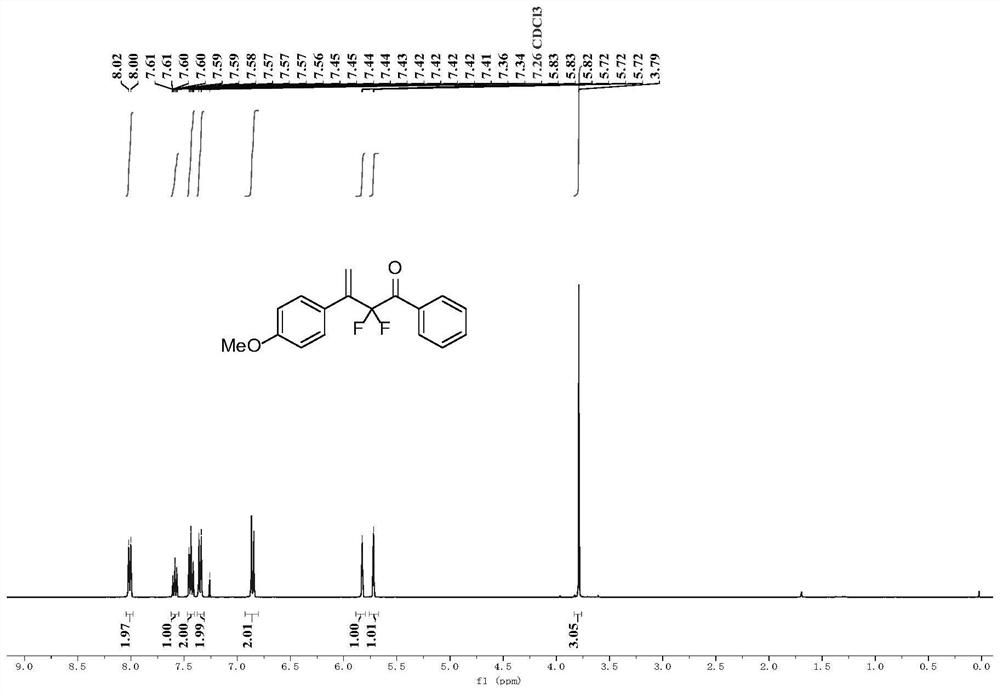
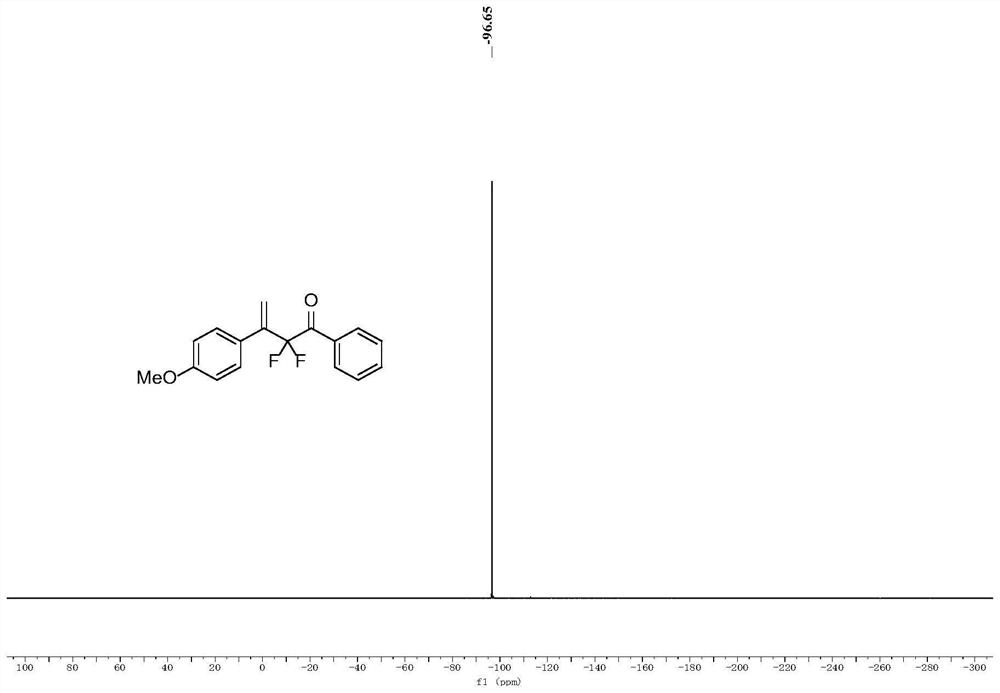
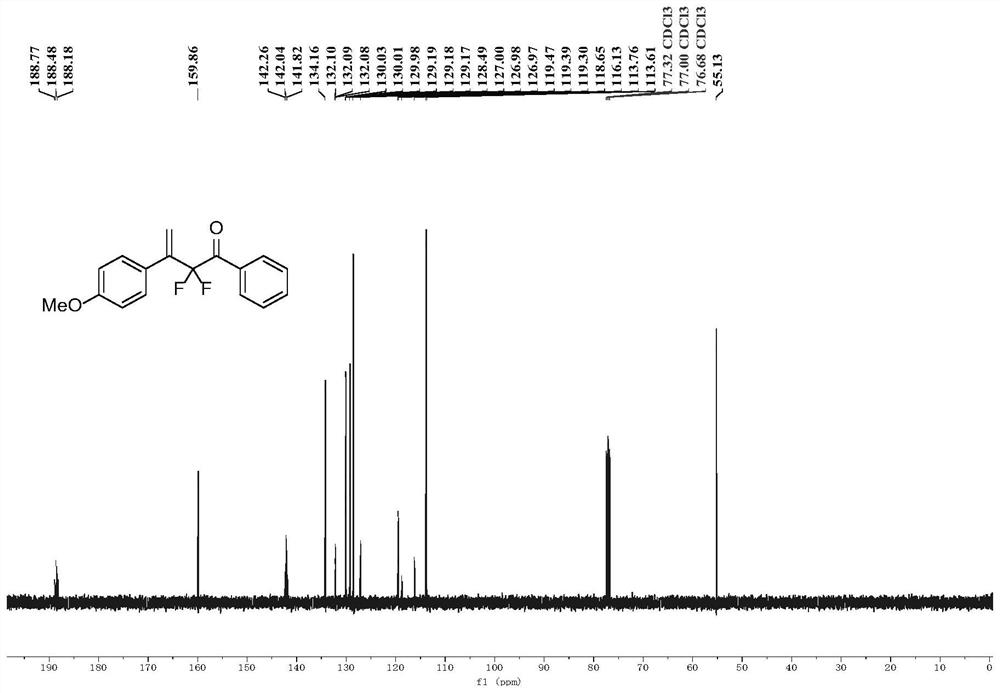
[0044] (1) Place a 20mL Schlenk tube equipped with a magnetic stirrer in an oven to dry for one hour, and add 0.2 g of Molecular sieves are then dried in an oven for half an hour, and after taking them out, they are hot and plugged with a rubber stopper and a nitrogen balloon. After cooling, ultra-dry 1,2-dichloroethane solvent (2 mL) was added thereto, followed by sealing the tube for nitrogen three times, and then adding 1-ethynyl-4-methoxybenzene (66.1 mg, 0.5mmol, 1equiv.), ((2,2-difluoro-1-phenylvinyl)oxy)trimethylsilane (172.2mg, 0.75mmol, 1.5equiv.), and ferric chloride ( 16.2mg, 0.1mmol, 0.2equiv.) and trimethylchlorosilane (108.7mg, 1mmol, 2equiv.). The mixture was stirred at -78°C for 12 hours.
[0045] (2) Remove the solvent by rotary evaporation subsequently, and the crude product is purified by silica gel column chromatography (column chromatography separation condition: the stationary phase is 300~400 mesh silica gel powder, and the mobile phase is ethyl aceta...
Embodiment 2
[0050] Example 2 is basically the same as Example 1, except that in step (1), the catalysts are different, as shown in Table 1 below.
[0051] Table 1
[0052] catalyst Yield(%) MgBr 2 (1 equiv.)
<10
AlCl 3 (1 equiv.)
<10
TiBr 4 (1 equiv.)
<10
CuBr 2 (1 equiv.)
<10
GaBr 3 (1 equiv.)
<10
InCl 3 (1 equiv.)
16 SnCl 4 (1 equiv.)
32 BiBr 3 (1 equiv.)
<10
FeCl 3 (1 equiv.)
57 FeBr 3 (1 equiv.)
34 FeBr 2 (1 equiv.)
<10
Fe(OTf) 3 (1 equiv.)
18 FeCl 3 (0.2 equiv.)
39 FeCl 3 (0.2equiv.), TMSCl (2equiv.)
79 TMSCl (2 equiv.) <5
[0053] As can be seen from Table 1, under the same reaction conditions, the use of catalysts, such as: magnesium bromide, aluminum trichloride, titanium tetrabromide, copper bromide, gallium bromide, indium trichloride, tin tetrachloride, Synthesis of 2,2-difluoro-3-(4-methoxyphenyl)-1-ph...
Embodiment 3
[0056] Example 3 is basically the same as Example 1, except that in step (1), the solvent and temperature are different, as shown in Table 2 below.
[0057] Table 2
[0058] solvent temperature(℃) Yield(%) Tetrahydrofuran -78℃ 0 1,4-dioxane -78℃ 0 N,N-Dimethylformamide -78℃ 0 Acetonitrile -78℃ 0 N,N-Dimethylacetamide -78℃ 0 n-Hexane -78℃ 0 Toluene -78℃ 0 Dichloromethane -78℃ 40 1,2-Dichloroethane -78℃ 79 1,2-Dichloroethane -40℃ 46 1,2-Dichloroethane 0℃ 41 1,2-Dichloroethane 60℃ 12
[0059] It can be seen from Table 3 that at -78°C, solvents containing heteroatoms, such as tetrahydrofuran, acetonitrile, N,N-dimethylformamide, N,N-dimethylacetamide, 1, Synthesis of 2,2-difluoro-3-(4-methoxyphenyl)-1-phenyl-3-en-1-one from 4-dioxane with very small yield; at -78°C , use a non-coordinating solvent, such as: toluene or n-hexane to synthesize the target product, with a very small y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com