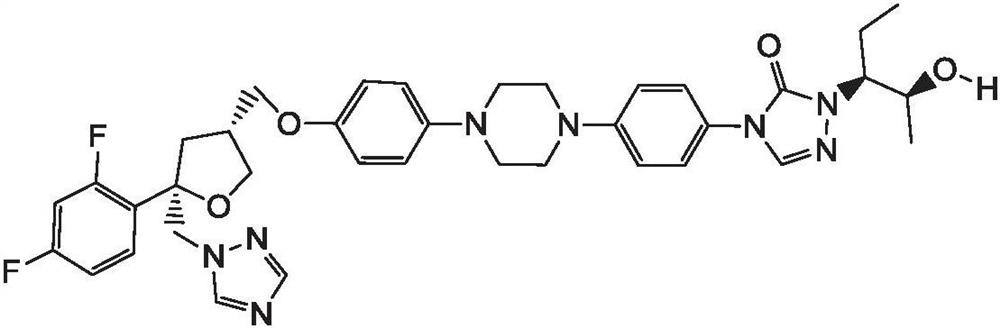
Synthesis method of 1-(4-aminophenyl)-4-(4-hydroxyphenyl) piperazine
A synthetic method, hydroxyphenyl technology, applied in organic chemistry, bulk chemical production, etc., can solve the problems of low yield, high price, and high production cost in the preparation process, and achieve strong operability, easy industrialization, and high purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
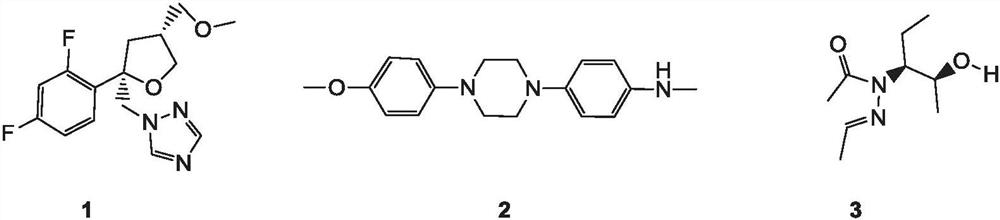
[0047] Step 1: Preparation of 4,4'-(piperazine-1,4-bis)phenol
[0048]
[0049] Add 50ml of ethanol, 1.723g (20mmol) of piperazine, 12.00g (60mmol) of p-iodophenol, 0.381g (2mmol) of cuprous iodide, 0.687g (4mmol) of metformin D, and 19.549g of cesium carbonate in a 100ml reaction bottle (60mmol), after the addition, the temperature was raised to reflux for 1 hour. After cooling down to room temperature, it was filtered, and the filtrate was concentrated to dryness and passed through a column to obtain 4.487 g of off-white solid (purity 98%, yield 83%). The experimental data of the hydrogen spectrum spectrum obtained by the nuclear magnetic hydrogen spectrum experiment are: 1 H NMR (400MHz, DMSO) δ6.85-6.73(m, 4H), 6.59-6.49(m, 4H), 3.66(s, 2H), 3.07(s, 8H).
[0050] Step 2: Preparation of 1-(4-acetylaminophenyl)-4-(4-hydroxyphenyl)piperazine
[0051]
[0052] Add 20ml of acetic acid, 2.703g (10mmol) of 4,4'-(piperazine-1,4-bis)phenol, 7.708g (100mmol) of ammonium ace...
Embodiment 2
[0057] Step 1: Preparation of 1,4-bis(4-((tetrahydropyran-2-yl)oxy)phenyl)piperazine
[0058]
[0059] Prepare a 100ml reaction bottle, pretreatment to keep dry and nitrogen blanketing, add ethylene glycol dimethyl ether 40ml, catalyst [Pd(IPr)Cl 2 ] 2 0.056g, 1.8g of potassium tert-amylate, 0.431g (5mmol) of piperazine, and 3.085g (12mmol) of p-bromophenoxytetrahydropyran, after the addition, the temperature was raised to 80°C for 1 hour to react. Then cool down to room temperature, add dropwise 5% ammonium chloride aqueous solution 80g below 20°C under temperature control, extract the reaction solution with 200g of dichloromethane, then concentrate to dryness, pass through the column to obtain 2.036g of off-white solid (purity 99%, yield 96%) ). The experimental data of the hydrogen spectrum spectrum obtained by the nuclear magnetic hydrogen spectrum experiment are: 1 H NMR (400MHz, DMSO) δ6.85-6.75(m, 4H), 6.70-6.60(m, 4H), 5.82(t, 2H), 3.20-2.96(m, 12H), 2.20-1.52(m,...
Embodiment 3
[0067] Step 1: Preparation of 1,4-bis(p-methoxyphenyl)piperazine
[0068]
[0069] Prepare a 100ml reaction bottle, pretreatment to keep dry and nitrogen blanketing, add toluene 40ml, catalyst palladium acetate 0.025g, potassium tert-amylate 1.8g, piperazine 0.431g (5mmol), p-methoxybromobenzene 2.244g (12mmol), heated up to 110°C for 1 hour after addition. Then cool down to room temperature, add dropwise 5% ammonium chloride aqueous solution 80g below 20°C under temperature control, extract the reaction solution with 200g of dichloromethane, then concentrate to dryness, pass through the column to obtain 1.343g of off-white solid (purity 99%, yield 90%) ). The experimental data of the hydrogen spectrum spectrum obtained by the nuclear magnetic hydrogen spectrum experiment are: 1 H NMR (400MHz, DMSO) δ6.85-6.75(m, 4H), 6.70-6.60(m, 4H), 3.33(s, 6H), 3.07(s, 8H).
[0070] Step 2: Preparation of 1,4-bis(p-hydroxyphenyl)piperazine
[0071]
[0072] Prepare a 50ml reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com