Two-dimensional MXene-inorganic-organic hybrid multifunctional hydrogel for promoting wound healing of infectious diabetes mellitus and preparation method of two-dimensional MXene-inorganic-organic hybrid multifunctional hydrogel
A technology of wound healing and organic hybridization, which is applied in the fields of pharmaceutical formulations, bandages, drug delivery, etc., can solve the problems of not being able to effectively improve the wound microenvironment, poor self-healing ability, and poor anti-infection ability, and achieve the purpose of inhibiting the growth of planktonic bacteria , Strong tissue adhesion, good anti-oxidation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Pluronic F127-methanesulfonate (F127-SO 3 ) preparation: 12.6g of F127 was dissolved in 120mL of anhydrous dichloromethane, then 0.88mL of triethylamine and 0.32mL of methanesulfonyl chloride were added successively, and the mixture was stirred at room temperature for 24h. After the reaction was complete, it was extracted with dichloromethane and the organic phase was washed with 1M hydrochloric acid and saturated sodium chloride. After drying with anhydrous sodium sulfate, it is precipitated in cold ether and dried in vacuo to obtain F127-SO 3 (89% yield).
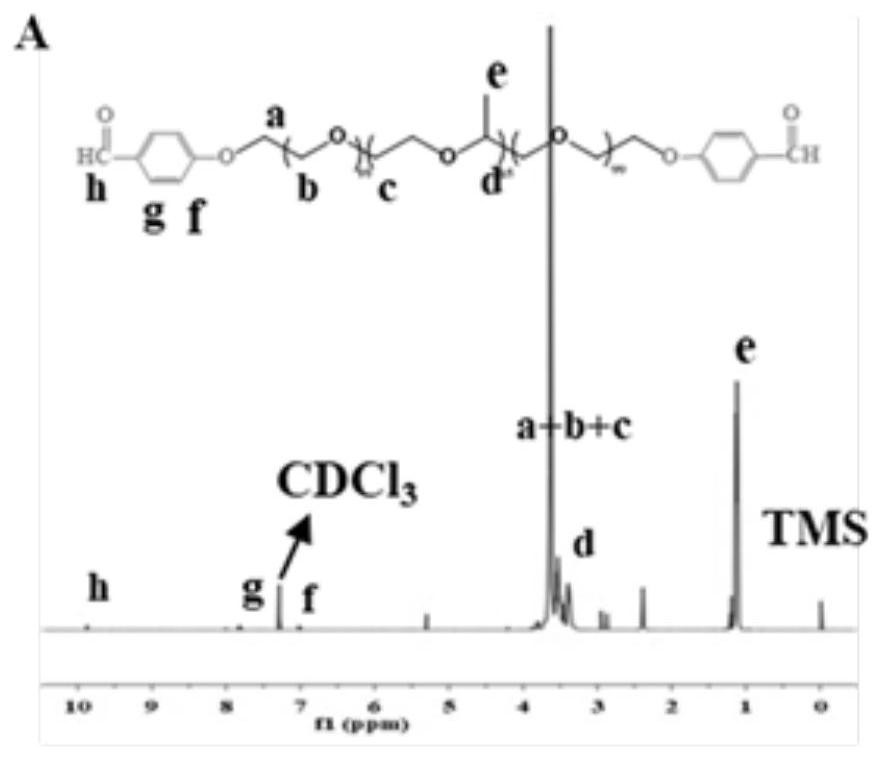
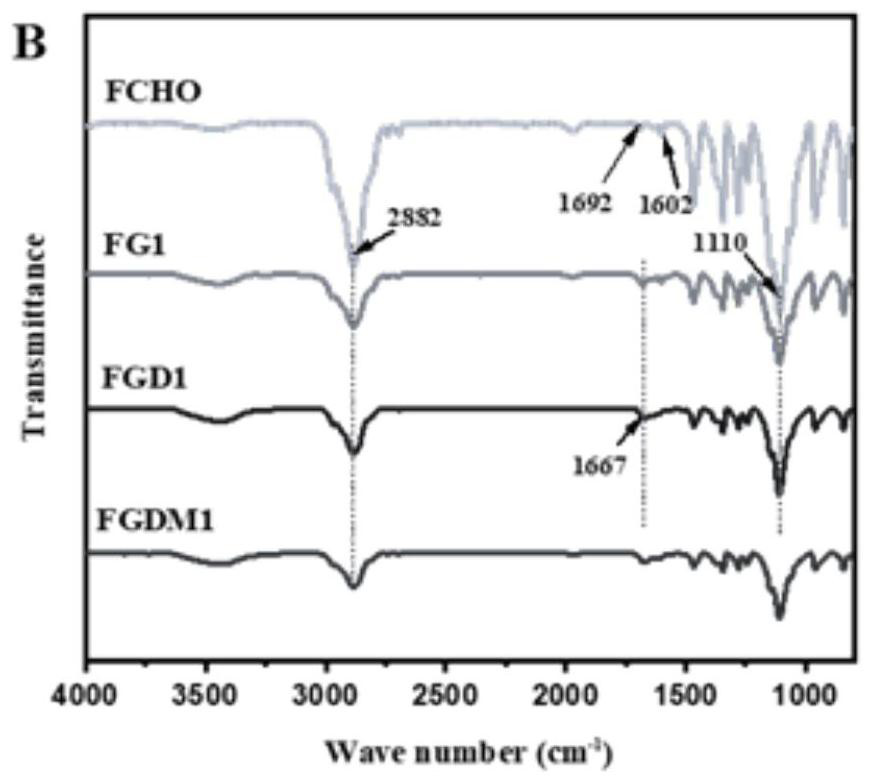
[0048] (2) Preparation of F127-p-hydroxybenzaldehyde (FCHO): 12.17g F127-SO 3 Dissolve in 120mL DMF, then add 0.52g p-hydroxybenzaldehyde and 0.59g potassium carbonate (K 2 CO 3 ), reacted at 80°C for 72h. After the reaction was completed, it was cooled to room temperature, 150 mL of water was added and extracted with dichloromethane. Then the organic phase was washed with saturated sodium chloride, dried...
Embodiment 2
[0056] (1) P123-methanesulfonate (P123-SO 3 ) preparation: 5.8g of P123 was dissolved in 70mL of anhydrous dichloromethane, then 0.56mL of triethylamine and 0.76g of methanesulfonyl chloride were sequentially added to react at room temperature for 24h. After the reaction was completed, it was extracted with dichloromethane and the organic phase was washed with dilute hydrochloric acid and saturated sodium chloride. After drying with anhydrous sodium sulfate, it is precipitated in cold ether and dried in vacuo to obtain P123-SO 3 (88% yield).
[0057] (2) Preparation of P123-p-hydroxybenzaldehyde (PCHO): 2.5g P123-SO 3 Dissolve in 40mL DMF, then add 0.15g p-hydroxybenzaldehyde and 0.16g potassium carbonate (K 2 CO 3 ) and reacted at 80°C for 72h. After the reaction was completed, it was cooled to room temperature, 50 mL of water was added and extracted with dichloromethane. Then, the organic phase was washed with saturated sodium chloride, dried over anhydrous magnesium s...
Embodiment 3
[0065] (1) Pluronic F127-methanesulfonate (F127-SO 3 ) preparation: 12.6g of F127 was dissolved in 120mL of anhydrous dichloromethane, then 0.88mL of triethylamine and 0.32mL of methanesulfonyl chloride were added successively, and the mixture was stirred at room temperature for 24h. After the reaction was complete, it was extracted with dichloromethane and the organic phase was washed with 1M hydrochloric acid and saturated sodium chloride. After drying with anhydrous sodium sulfate, it is precipitated in cold ether and dried in vacuo to obtain F127-SO 3 (87% yield).
[0066] (2) Preparation of F127-p-hydroxybenzaldehyde (FCHO): 12.17g F127-SO 3 Dissolve in 120mL DMF, then add 0.52g p-hydroxybenzaldehyde and 0.59g potassium carbonate (K 2 CO 3 ), reacted at 80°C for 72h. After the reaction was completed, it was cooled to room temperature, 150 mL of water was added and extracted with dichloromethane. Then, the organic phase was washed with saturated sodium chloride, drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com