Method for improving oxygen evolution reaction performance of layered metal hydroxide
A technology of hydroxides and layered metals, applied in the field of electrochemistry, can solve the problems of high price, limited wide application, and low reserves of precious metal catalysts, and achieve the effect of optimizing activity and stability and improving activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
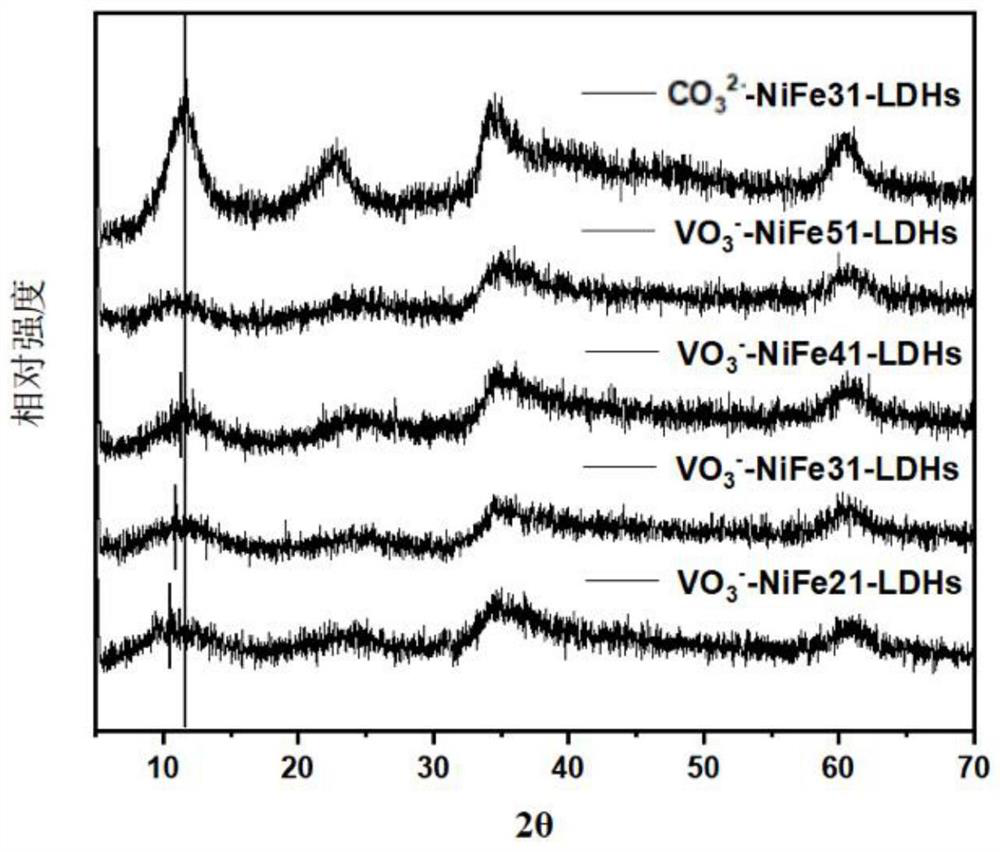
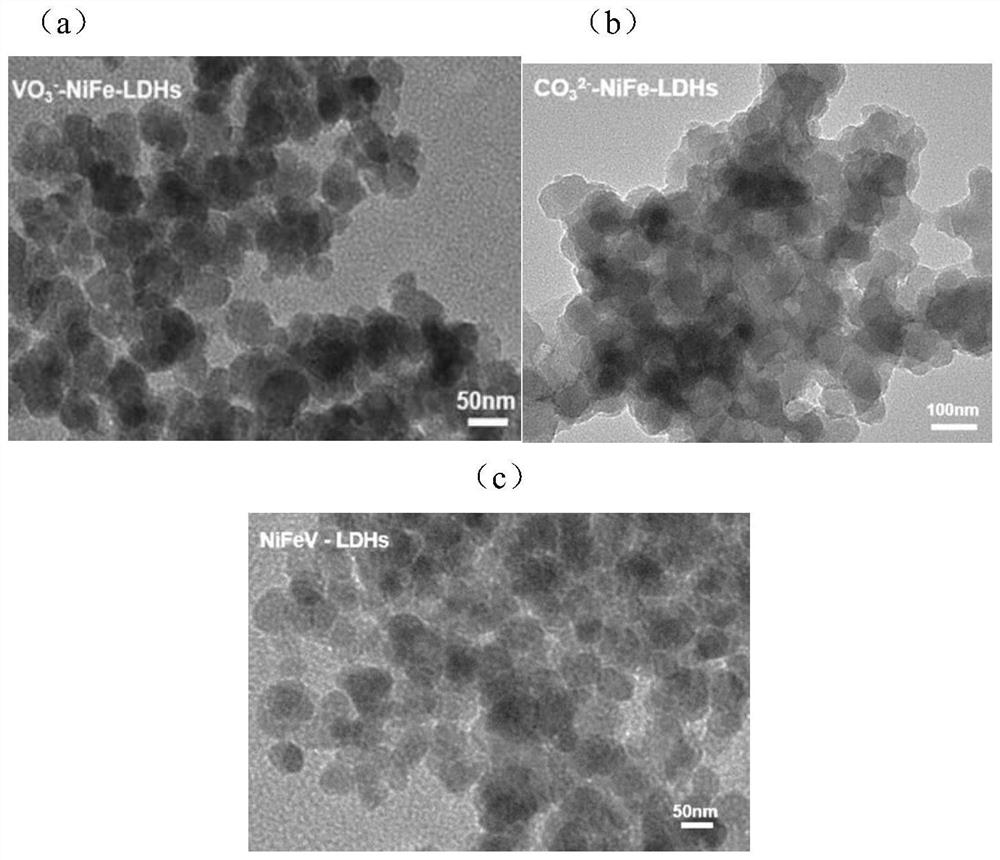
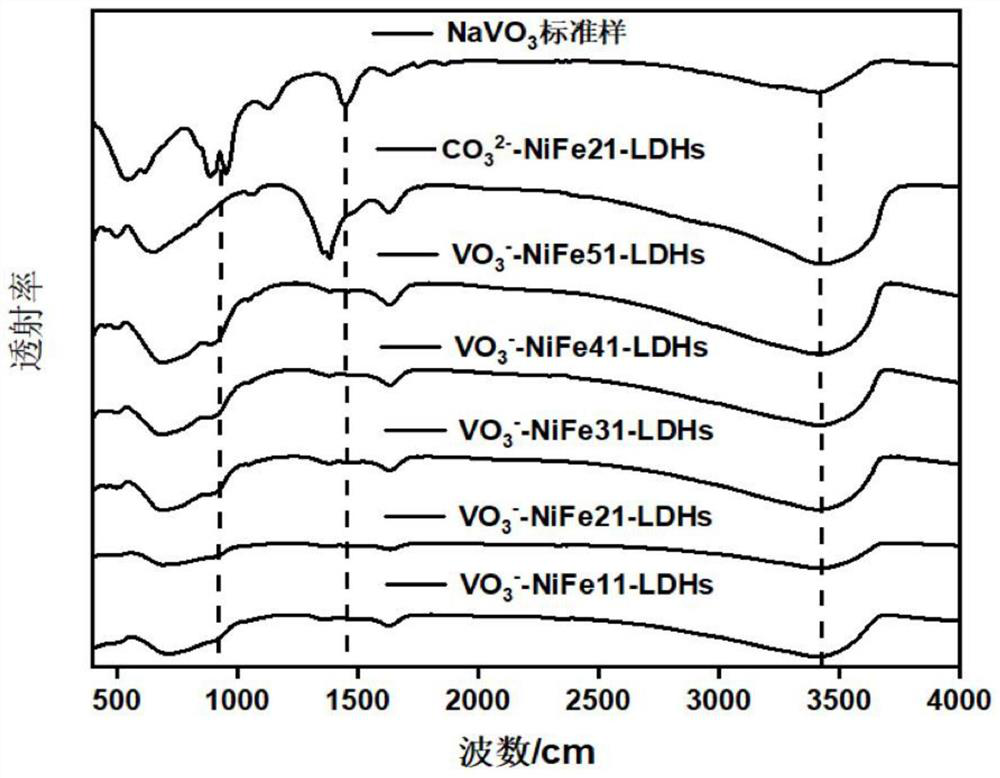
[0042] Preparation of Layered Metal Hydroxide-Metavanadate Intercalated NiFe-LDHs
[0043] Metavanadate ion intercalated NiFe-LDH was prepared by coprecipitation method. Different ratios of nickel nitrate hexahydrate and ferric nitrate nonahydrate were made into 30 mL solutions (nickel-iron molar ratio 1:1 to 5:1), which was defined as solution A. Dissolve 0.6 g of sodium hydroxide in 30 mL of aqueous solution, which is defined as solution B; solution C is a solution of 0.6 g of sodium metavanadate dissolved in 30 mL of deionized water (the water used in the experiment is deionized water protected by nitrogen) .
[0044] Add solution A and B to solution C dropwise, stir while passing nitrogen, stir in 80° C. water bath for 20 min, keep pH~10, form a solution with yellow solid precipitation, and wash the suspension obtained above by centrifugal washing ( Alternate cleaning with deionized water and ethanol solution, due to freeze-drying, the last pass must be deionized water c...
Embodiment 2
[0046] Preparation of layered metal hydroxide-carbonate intercalated NiFe-LDHs:
[0047] Preparation of CO by the Double Drop Method 3 2- Intercalated Ni 2 Fe 1 -LDHs catalyst. Specifically, the preparation strategy of the double-drop method is as follows: 1.2 mmol of nickel nitrate hexahydrate and 0.6 mmol of ferric nitrate nonahydrate are dissolved in 30 mL of deionized water. It was named Mixed Solution A. Dissolve 0.6 g of sodium hydroxide and 0.6 g of sodium carbonate in 30 mL of deionized water, thoroughly mix and dissolve after 5 min of ultrasound, and finally obtain a clear dispersion liquid, which is named mixed solution B. Dissolve 0.6 g of sodium carbonate in 30 mL of deionized water, and make it fully mixed and dissolved after 5 minutes of ultrasonication to finally obtain a clear dispersion liquid, which is named mixed solution C. The mixed solution A and the mixed solution B were slowly and uniformly dropped into the stirred solution C at the same time, kee...
Embodiment 3
[0049] Preparation of layered metal hydroxide-carbonate intercalated NiFeV-LDHs:
[0050] A series of NiFeV-LDHs catalysts with different NiFeV ratios were prepared by the double-drop method. The preparation strategy of the double drop method is: three proportions of ternary NiFeV hydrotalcite need to be prepared in this experiment, namely Ni 2 Fe 1 V 0.5 -LDHs, Ni 2 Fe 1 V 1.0 -LDHs, Ni 2 Fe 1 V 1.5 -LDHs. with Ni 2 Fe 1 V 0.5The preparation method of -LDHs is taken as an example, 2 mmol of nickel nitrate hexahydrate, 1 mmol of ferric nitrate nonahydrate, 0.5 mmol of vanadium trichloride are dissolved in 30 mL of deionized water, and it is fully mixed and dissolved by ultrasonic for 5 min, and finally a clear dispersion liquid is obtained. , named as mixed solution A; dissolve 0.6 g of NaOH in 30 mL of deionized water, and make it uniformly dispersed after 5 min of ultrasound, and named it as mixed solution B; solution C is 30 mL of deionized water. The mixed sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com