Cationic peptide C9 and application thereof
A cationic peptide, Gram-positive technology, applied in the field of protein engineering, can solve the problems of low protease stability and limited clinical application, and achieve increased depolarization level, good antibacterial activity in vitro, and anti-biofilm formation ability is not easy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
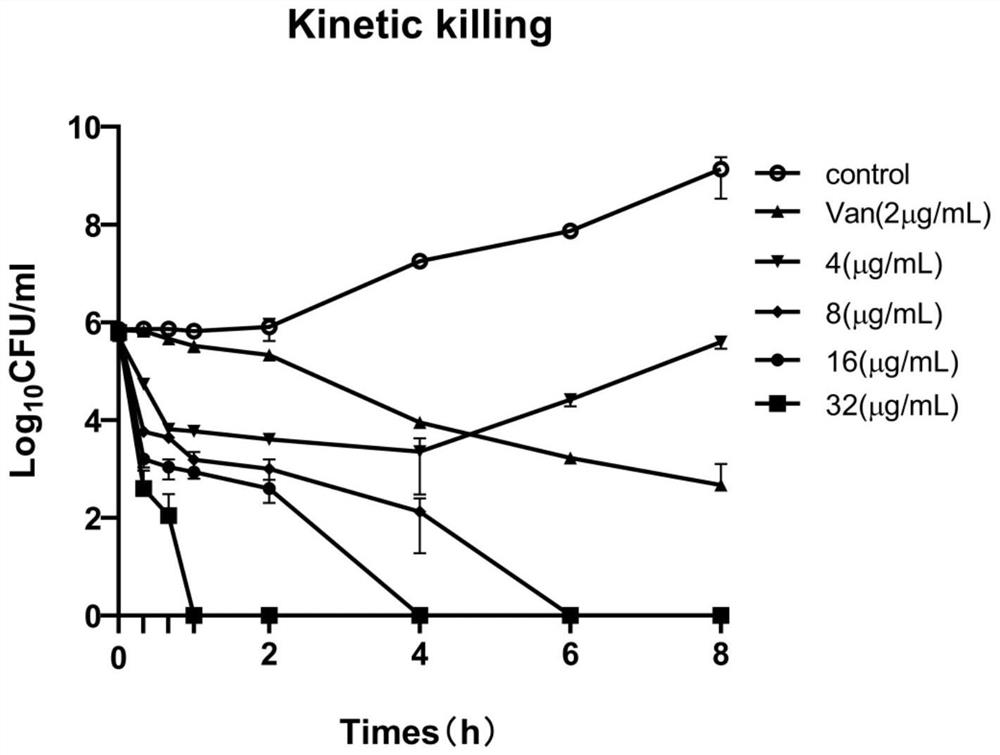
[0044] Example 1: In vitro biological activity of C9
[0045] 1. Experimental strains
[0046] 34 clinical samples of Staphylococcus epidermidis were isolated, and duplicate samples were excluded from the same patient. Sample source: sputum, blood, urine, etc. Collection and use of clinical isolates have been approved by the Institutional Review Board (IRB) of Guizhou Medical University, and studies involving human participants have been reviewed and approved by Guizhou Medical University and the ethics committee affiliation. Bacterial identification and drug susceptibility tests were carried out by French bio-Mérieux VITEK-2 automatic microbiological analyzer. Staphylococcus epidermidis ATCC35984 was a positive strain for biofilm formation, and the above strains were preserved by the Laboratory of Pathogen Biology of Guizhou Medical University.
[0047] 2. Method
[0048] 2.1 Minimum inhibitory concentration (MIC) determination
[0049] 1) Preparation of bacterial cells:...
Embodiment 2
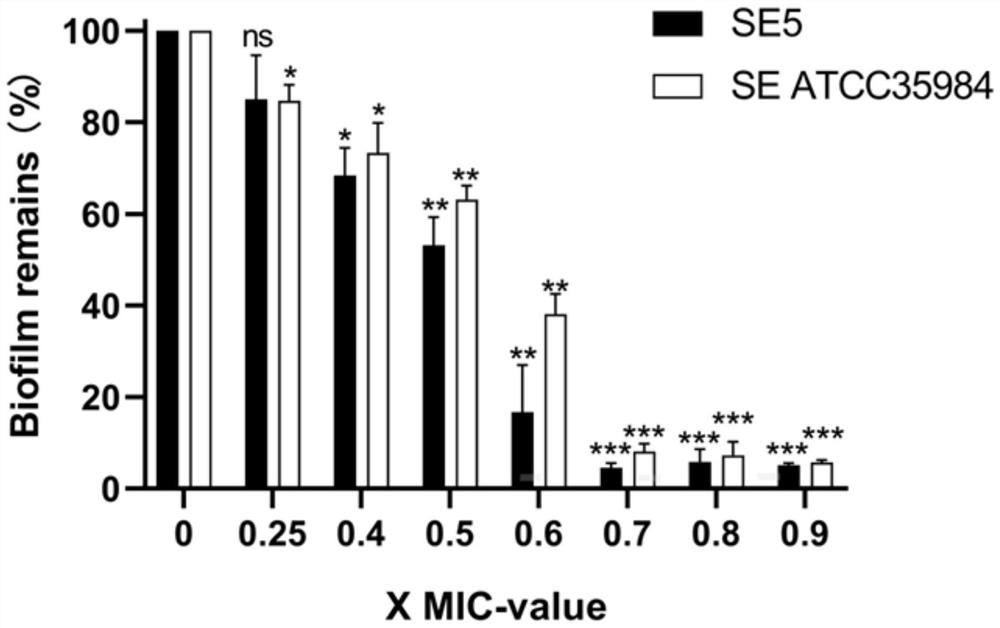
[0086] Example 2: Effect of C9 on Staphylococcus epidermidis biofilm
[0087] 1. Experimental strains
[0088] Staphylococcus epidermidis ATCC 35984 and clinical isolates (SE5).
[0089] 2. Method
[0090] 2.1 Biofilm forming ability
[0091] Take the logarithmic growth phase of Staphylococcus epidermidis and prepare the concentration of 2 × 10 in TSB medium. 6 CFU / mL bacterial suspension. 200 μL of bacterial solution was added to each well of a 96-well plate, TSB medium was used as a negative control, and three replicate wells were set up for each bacterial strain. After static culture at 37 °C for 24 h, the 96-well plate was taken out, non-adherent bacteria and cultures were discarded, the culture wells were washed three times with sterile PBS, the plate was placed in a 60 °C incubator to dry and fix for 30 min, and 200 μL was added to the wells after fixation. 0.1% crystal violet (CV), shaking at room temperature for 20 min; discarding the crystal violet solution, gent...
Embodiment 3
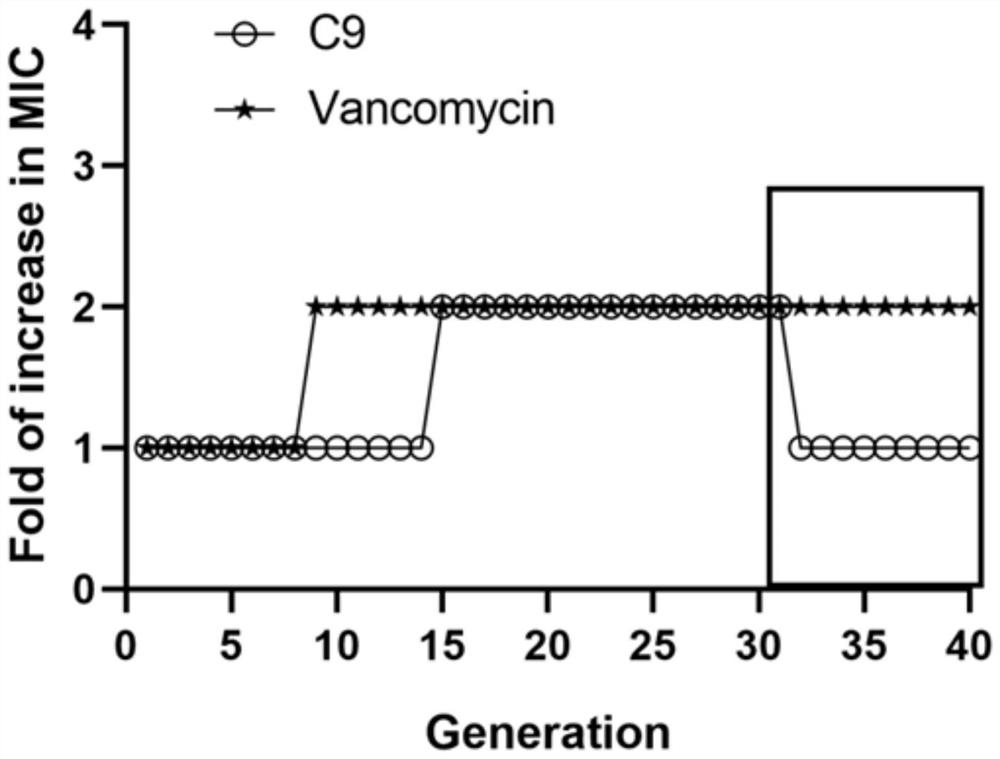
[0107] Example 3: Research on the bactericidal mechanism of C9
[0108] 1. Experimental strains
[0109] Staphylococcus epidermidis ATCC 35984 and clinical isolates (SE5).
[0110] 2. Experimental method
[0111] 2.1 Microscopic observation of morphological changes
[0112] 1) Laser confocal microscope
[0113] In order to verify whether C9 can bind to the cell membrane of S. epidermidis through electrostatic interaction, the labeling of S. epidermidis by FITC-C9 was observed by laser confocal microscopy.
[0114] Take log phase Staphylococcus epidermidis and prepare 1 mL with PBS to a concentration of 1 × 10 8 CFU / mL bacterial suspension was added with FITC-C9 (1×MIC) peptide solution and incubated in a 37°C incubator for 1 h. After the incubation, the cells were collected by centrifugation at 4000 rpm for 5 min, washed three times with PBS to remove unbound peptides, and finally resuspended in 500 μL of PBS. Coverslip and seal, and observe with a laser confocal microsc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com