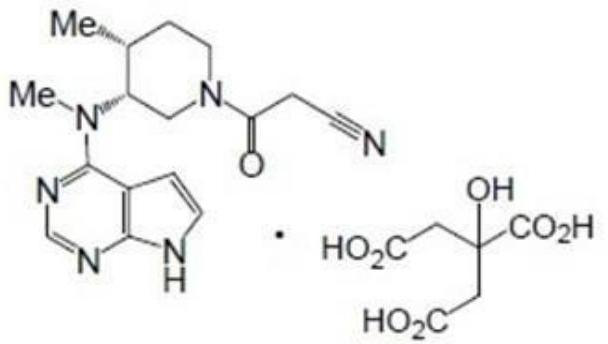
Tofacitinib gastric retention floating sustained release tablet and preparation method thereof
A technology of tofacitinib and gastric retention, which is applied in the field of medicine, can solve the unsolved problems such as low absorption in the lower part of the digestive tract, and achieve the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
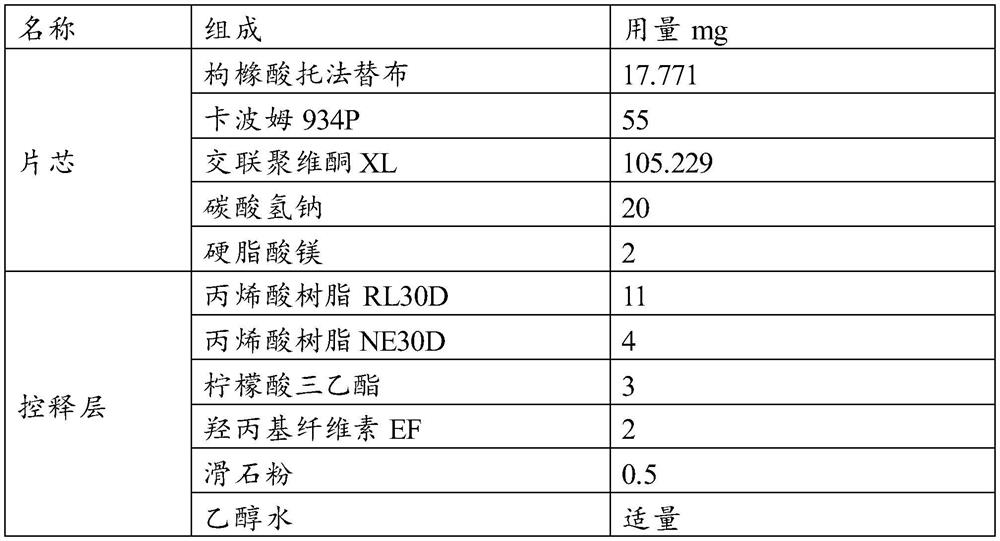
[0042]
[0043] Preparation Process:
[0044] (1) get tofacitinib citrate, crospovidone XL, sodium bicarbonate, carbomer 934P, magnesium stearate and cross 40 mesh sieves respectively, for subsequent use;
[0045] (2) Weigh tofacitinib citrate, crospovidone XL (about 1 / 2 of the recipe) and carbomer 934P and place them in the hopper, mix them evenly, and then add the remaining crospovidone XL and sodium bicarbonate, mix evenly, and finally add magnesium stearate, mix evenly, to obtain the final mixed material;
[0046] (3) placing the above-mentioned homogeneously mixed particles in a rotary tableting machine, tableting, and obtaining tofacitinib citrate sustained-release tablet cores with a hardness of 70 to 110N;
[0047] (4) preparing a coating solution, and then the prepared tablet core is coated with a controlled release film, and the coating weight gain is about 7%;
[0048] (5) Using the aluminum-plastic blister packaging machine, the above-mentioned coated tablets ...
Embodiment 2
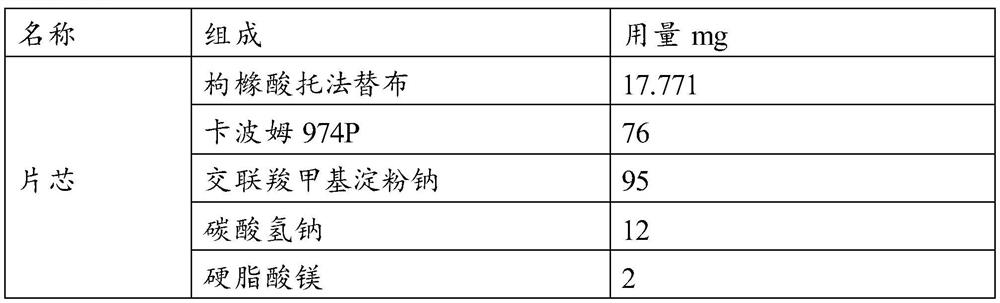
[0050]
[0051]
[0052] Preparation Process:
[0053] (1) get tofacitinib citrate, croscarmellose sodium starch, sodium bicarbonate, carbomer 974P, magnesium stearate and cross 40 mesh sieves respectively, for subsequent use;
[0054] (2) Weigh tofacitinib citrate, sodium cross-linked carboxymethyl starch (about 1 / 2 of the recipe), and carbomer 974P and place them in the hopper, mix them evenly, and then add the remaining cross-linked carboxymethyl Sodium starch and sodium bicarbonate, mix well, finally add magnesium stearate, mix well, and get the final mixed material;
[0055] (3) placing the above-mentioned homogeneously mixed particles in a rotary tableting machine, tableting, and obtaining tofacitinib citrate sustained-release tablet cores with a hardness of 70 to 110N;
[0056] (4) preparing a coating solution, and then the prepared tablet core is coated with a controlled release film, and the coating weight gain is about 7%;
[0057] (5) Using the aluminum-plas...
Embodiment 3
[0059]
[0060] Preparation Process:
[0061] (1) get tofacitinib citrate, sodium carboxymethyl starch, sodium bicarbonate, carbomer 974P, magnesium stearate and cross 40 mesh sieves respectively, for subsequent use;
[0062] (2) Weigh tofacitinib citrate, sodium carboxymethyl starch (about 1 / 2 of the recipe), and carbomer 974P and place them in the hopper, mix them evenly, and then add the remaining sodium carboxymethyl starch and carbonic acid. Sodium hydrogen, mix evenly, finally add magnesium stearate, mix evenly, to obtain a total mixed material;
[0063] (3) placing the above-mentioned homogeneously mixed particles in a rotary tableting machine, tableting, and obtaining tofacitinib citrate sustained-release tablet cores with a hardness of 70 to 110N;
[0064] (4) preparing a coating solution, and then the prepared tablet core is coated with a controlled release film, and the coating weight gain is about 7%;
[0065] (5) Using the aluminum-plastic blister packaging m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com