Method for preparing olefin-containing ester compound under catalysis of deep eutectic solvent
A technology for deep eutectic solvents and ester compounds, which is applied in the field of deep eutectic solvent catalyzed preparation of olefin-containing ester compounds, can solve the problems of difficult recovery, corrosion side reactions, poor catalyst stability, etc. Catalytic efficiency, good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Synthesis of Lauryl Sorbate
[0062] In a 100mL single-neck flask, add oxalic acid (7.2g, 0.08mol), tetraethylammonium chloride (13.3g, 0.08mol), p-tert-butylcatechol (0.14g, 1%) and stir at 80°C for 30min until The system became a colorless and transparent liquid to obtain a deep eutectic solvent, and then sorbic acid (10.76g, 0.096mol) and lauryl alcohol (18.2mL, 0.08mol) were added, and the reaction was carried out at 90 ° C for 8h. After the reaction, the layers were separated and cooled. The organic layer was taken away to be lauryl sorbate with a yield of 83%. New raw materials are added to the remaining deep eutectic solvent layer, and the above process is repeated, and the yield is still more than 80% after the deep eutectic solvent is recycled for 10 times.
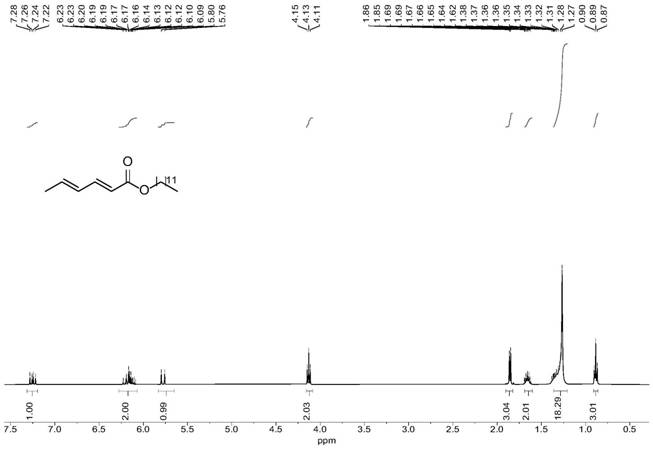
[0063] Lauryl Sorbate
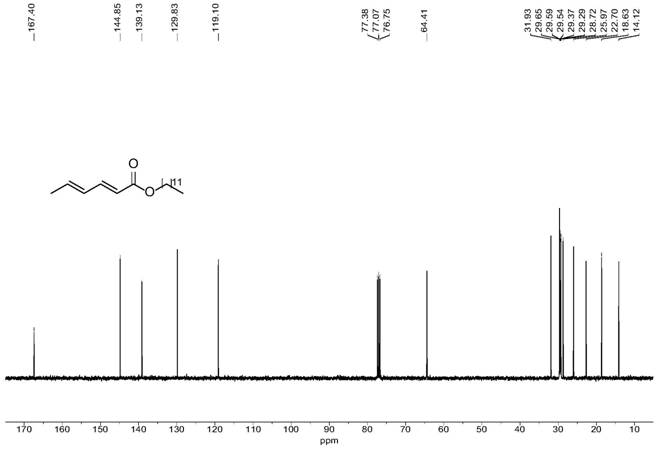
[0064] colorless liquid; 1 H NMR (400MHz, Chloroform-d) δ 7.25 (dd, J=15.4, 10.1 Hz, 1H), 6.23–6.09 (m, 2H), 5.78 (d, J=15.4 Hz, 1H), 4.13 (t, J=6.7Hz, 2H), 1.85 (d...
Embodiment 2
[0065] Example 2: Synthesis of Lauryl Cinnamate
[0066] In a 100mL single-neck flask, add citric acid (15.4g, 0.08mol), choline chloride (5.6g, 0.08mol), 3,5-di(tert-butyl)catechol (0.18g, 1%) Stir at 80°C for 30min until the system becomes a colorless and transparent liquid to obtain a deep eutectic solvent, then add cinnamic acid (14.2g, 0.096mol), lauryl alcohol (18.2mL, 0.08mol), and react at 90°C for 8h, the reaction After finishing layering, taking away the organic layer after cooling is lauryl cinnamate, the yield is 98%, adding new raw materials in the remaining deep eutectic solvent layer, and repeating the above process, the deep eutectic solvent is recycled 10 times The post-yield is still over 90%.
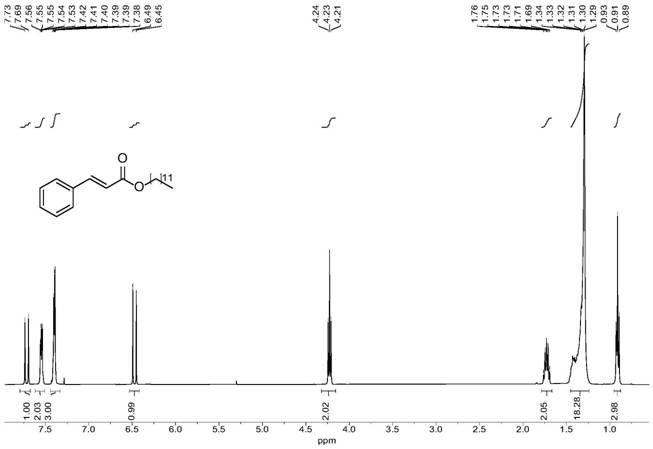
[0067] Lauryl Cinnamate
[0068] colorless liquid; 1 H NMR (400MHz, Chloroform-d) δ 7.71 (d, J=16.0Hz, 1H), 7.56–7.53 (m, 2H), 7.42–7.38 (m, 3H), 6.47 (d, J=16.0Hz, 1H), 4.23 (t, J=6.7Hz, 2H), 1.76–1.69 (m, 2H), 1.34–1.29 (m, 18H), 0.97–0.88 (t, J=6.5Hz, 3H); 1...
Embodiment 3
[0069] Example 3: Synthesis of Dilauryl Maleate
[0070] In a 100mL single-necked flask, add trichloroacetic acid (13.1g, 0.08mol), tetrabutylammonium chloride (11.1g, 0.04mol), bisphenol A (0.1g, 1%) and stir at 50°C for 30min until the system It became a colorless and transparent liquid to obtain a deep eutectic solvent, and then maleic acid (4.65g, 0.04mol) and lauryl alcohol (22.7mL, 0.1mol) were added, and the reaction was carried out at 90 ° C for 8h. After the reaction, the layers were separated. The upper organic layer is distilled under reduced pressure (-100KPa) at 110°C to steam lauryl alcohol and reclaim to obtain the product dilauryl maleate yield 92%, and the steamed lauryl alcohol is dropped into a deep eutectic solvent to continue the reaction, Adding new raw materials and repeating the above process, the yield is still more than 80% after the deep eutectic solvent is recycled for 10 times.
[0071] Dilauryl Maleate
[0072] colorless liquid; 1 H NMR (400M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com