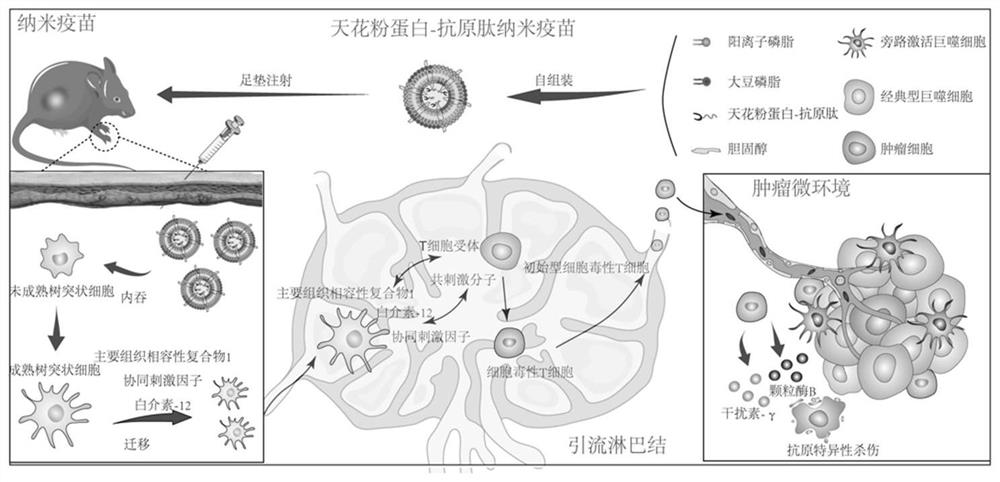
Cationic liposome vaccine as well as preparation method and application thereof
A cationic liposome and cationic lipid technology, applied in the field of biomedicine, can solve the problems of low immunogenicity, disappearance, and high cost of tumors, and achieve inhibition of tumor growth and spread, activation and proliferation, and promotion of recognition and presentation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
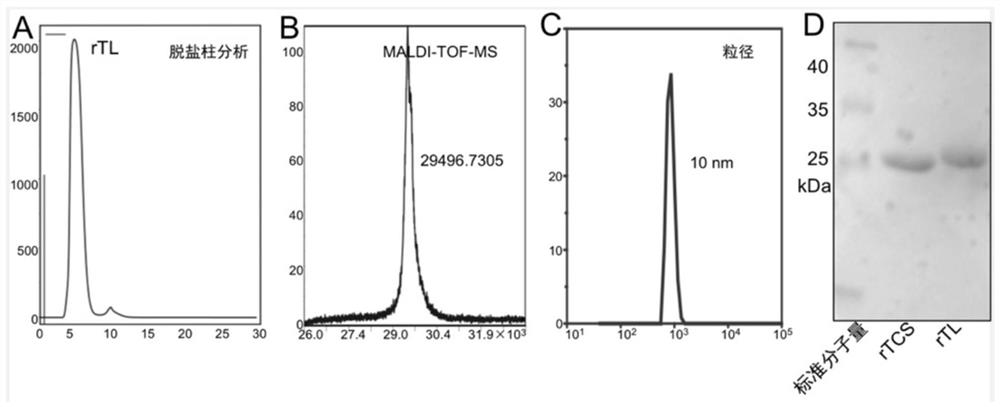
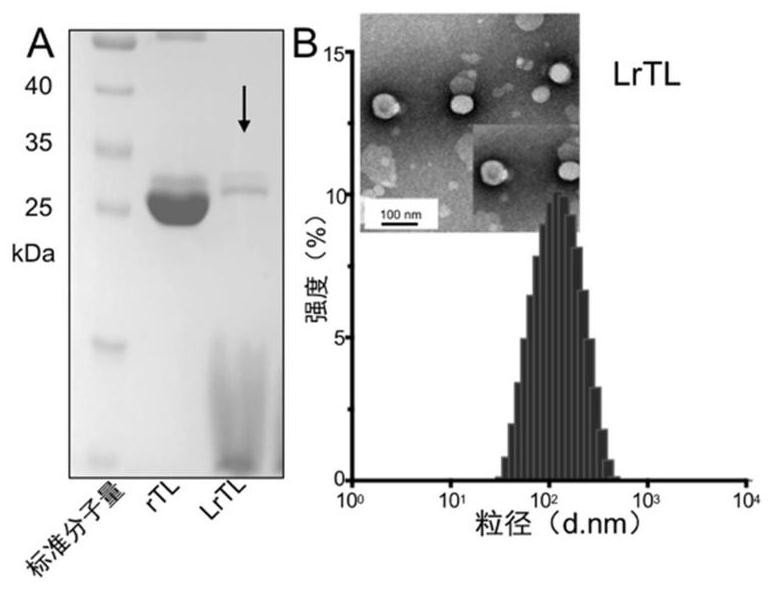
[0045] Preparation Example 1: Preparation of fusion protein 1 (rTCS-LEG(rTL))
[0046] Prokaryotic expression and purification of TCS-antigen peptide protein
[0047] a: The plasmid pET28a-TCS-legumain was transformed into E. coli BL21(DE3) competent cells.
[0048] b: Take 100 μL of transformed strains into 2 mL of LB medium containing 50 μg / mL Amp, cultivate at 37°C, 250 rpm for 3-4 h, and cultivate to logarithmic growth phase (absorbance at 600 nm is 0.6-0.8), adding the final concentration of 1 mM The IPTG was expressed overnight (16h) at 25°C, 150rpm.
[0049] c: Freeze centrifugation (9000rpm, 4°C, 3min) to collect the cells.
[0050] d: The bacteria were resuspended with HEPES buffer (containing 20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5‰ Tween 20, pH 8.5).
[0051] e: Probe ultrasonic instrument to break the bacteria (over 3s, stop for 3s, power 400W, 40min, ice bath), until the bacteria liquid is clear and translucent.
[0052] f: centrifugal treatment of bacterial ...
preparation Embodiment 2
[0057] Preparation Example 2: cy5-labeled recombinant trichosanthin-antigen peptide vaccine
[0058] 1) cy5-NHS ester labeled rTL
[0059] Weigh a certain amount of cy5 and dissolve it in ultrapure water. According to the molar concentration ratio of rTL:cy5=1:3, slowly drop the cy5 solution into rTL, vortex while adding dropwise, and place it at 4°C to react overnight in the dark. , and then separated with a desalting column to remove unreacted cy5. The collected rTL-cy5 was ultrafiltered with an ultrafiltration tube with a molecular weight cut-off of 10 kDa, concentrated to 1.5 mL, and the amount of cy5 was determined by a fluorescence spectrophotometer, and the device was stored at -20 °C for use.
[0060] 2) rTL-cy5 standard curve
[0061] Fluorescence quantification of rTL-cy5 was performed by spectrofluorophotometry. Aspirate 50 μL of rTL-cy5 with a micropipette and dilute to 2 mL with water. After gradient dilution, the cy5 fluorescence intensity of samples with dif...
preparation Embodiment 3
[0062] Preparation Example 3: Preparation of Cationic Liposome Vaccine
[0063] 1) Preparation of cationic liposome membranes
[0064] Weigh 20 mg of soybean lecithin SPC-100, dissolve with chloroform, ultrasonicate for 10 min to make the final concentration 20 mg / mL; weigh 5 mg of cholesterol, dissolve in chloroform, ultrasonicate for 10 min, the final concentration is 5 mg / mL, weigh DOTAP 10 mg, dissolved in chloroform to a final concentration of 1 mg / mL. Take 1 mL of soybean phospholipid SPC-100 solution, add 1 mL of cholesterol solution, 0.4 mL of DOTAP solution, sonicate in a water bath for 20 seconds, mix well, add to a 250 mL round-bottomed flask, and spin dry the above solvent with a rotary evaporator at 37 °C , put it in a vacuum drying box to dry overnight to form a cationic liposome membrane.
[0065] 2) Add 1 mL of rTCS-LEG aqueous solution, add steel balls to hydrate for 10 min at room temperature, and continue to add 4 mL of ultrapure water to fully hydrate for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com