Application of Lpp or mutant thereof as molecular chaperone to secretory expression of recombinant protein in escherichia coli
A technology of Escherichia coli, secreted expression, applied in the field of biomedicine, which can solve the problems of low proportion of target protein, increased probability of miscutting, and more impurities, and achieve the effect of high proportion, increased resistance, and increased production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The technical service company synthesized the gene sequence, and its translated amino acid sequence had the following characteristics as shown in Table 1:
[0051] Table 1
[0052]
[0053]
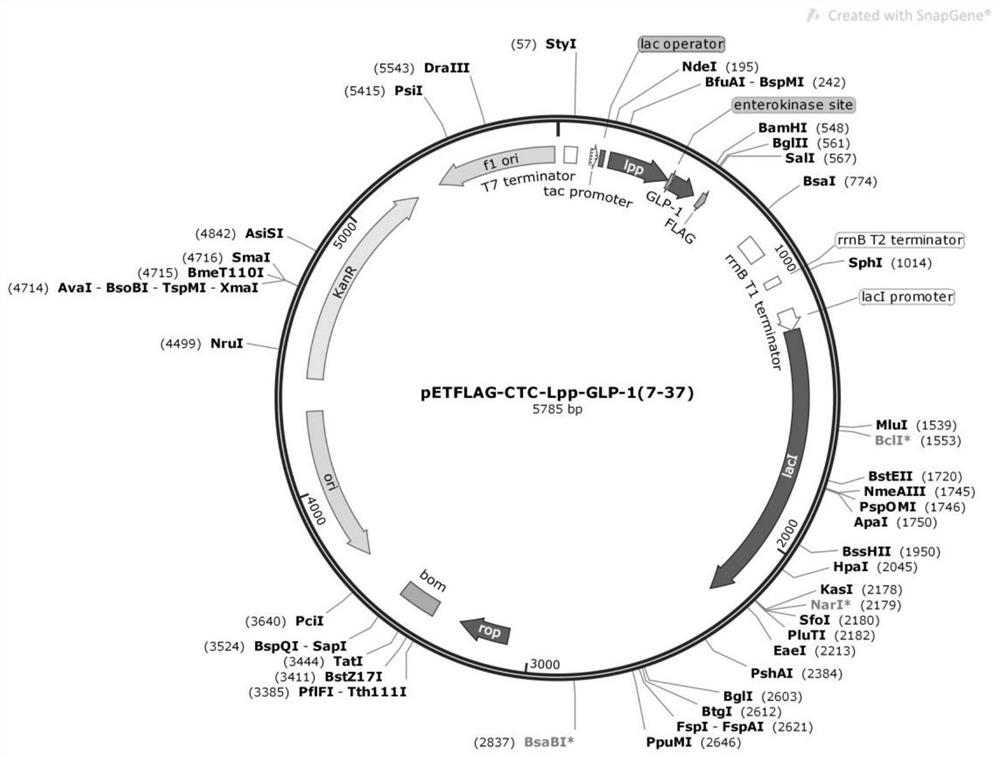
[0054] Transformation of pET-28a(+) vector: PCR amplification of the tac promoter region of pFLAG-CTC vector (Sigma), adding BlpI and SphI restriction sites at both ends of the primers, BlpI and SphI double restriction PCR products and pET-28a (+) The vector was ligated with T4 DNA ligase and transformed into E. coli top10 competent cells, and positive clones were identified by PCR. After the plasmid was extracted, a promoter-replaced pET-28a(+) vector was obtained, which was named pETFLAG-CTC.
[0055] The gene sequence encoding the above-mentioned amino acid sequence was synthesized with reference to the codon bias of Escherichia coli (as shown below, synthesized by a technical service company). Taking SEQ ID NO.4 as an example, BamHI and NdeI restriction sites were added...
Embodiment 2
[0062] Example 2 Escherichia coli transformation and screening
[0063] The recombinant expression vector obtained in Example 1 was transformed into Escherichia coli for replication and amplification, and the specific process was as follows: according to the calcium chloride method (refer to the third edition of "Molecular Cloning Experiment Guide"), Escherichia coli top10 competent state was prepared, and 1 μL of the recombinant expression vector was taken. Add to top10 competent, ice bath for 30min, heat shock at 42°C for 90s, ice bath for 5min, add 1ml liquid SOC medium (2%w / v tryptone, 0.5%w / v yeast extract, 0.05%w / v NaCl, 2.5 mM KCl, 10 mM MgCl 2 , 20 mM glucose), incubate at 37 °C for 1 h after shaking in a shaker, spread on LB solid medium (containing 50 mg / L kanamycin kan), and cultivate overnight in a 37 °C incubator until colonies are visible to the naked eye. Pick bacteria into LB liquid medium (10g / L peptone, 5g / L yeast extract, 5g / L sodium chloride, pH7.0~7.5, co...
Embodiment 3
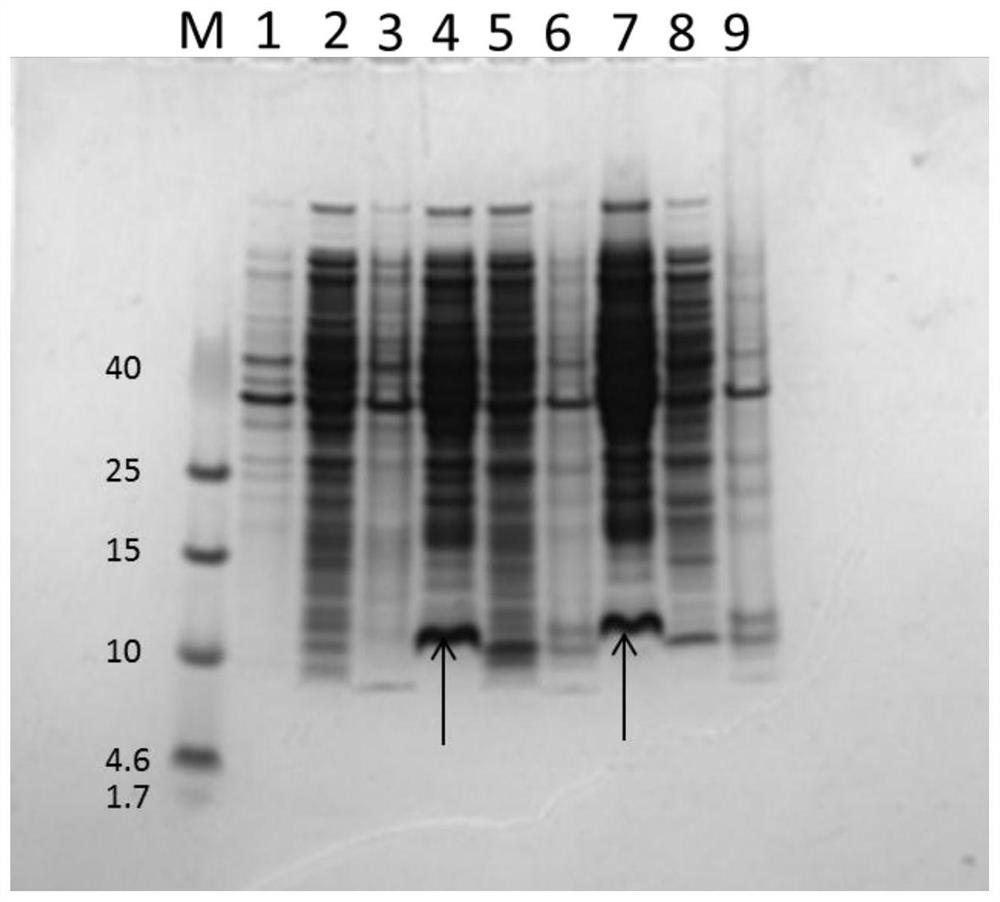
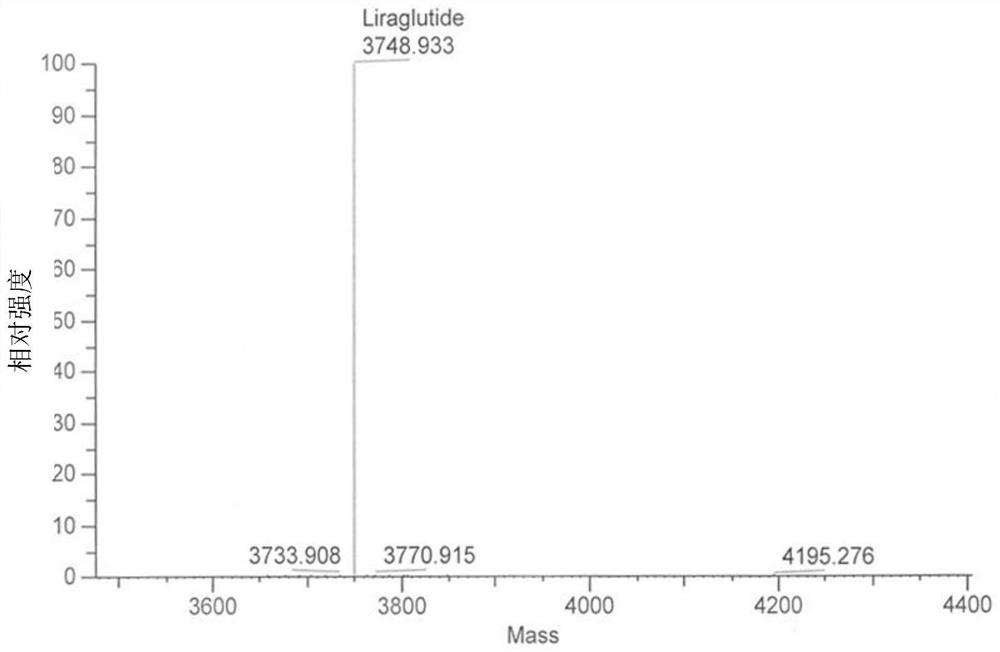
[0065] Example 3. Escherichia coli fermentation and purification
[0066] The bacterial classification that embodiment 2 preserves prepares seed culture and 20L fermentor fermentation process:
[0067] ① Preparation of seed culture
[0068] Take 20 μL of the strains BL21(DE3) / pETFLAG-CTC-Lpp-GLP-1(X) and W3110 / pETFLAG-CTC-Lpp-GLP-1(X) cryopreserved at -70℃, respectively, inoculate to 50 mL with added The LB liquid medium of kanamycin (final concentration of 50 μg / mL) was cultured at 28° C. and 250 rpm in a shaker for 16 hours to activate the strains. Then inoculate 50 mL of activated strains into 400 mL of LB liquid medium supplemented with 50 μg / mL kanamycin, and continue to cultivate for 3 hours at 28 °C and 250 rpm to obtain a seed culture and control its bacterial concentration. OD600 is between 0.8 and 1.2.
[0069] ②Fermentation culture in 20L fermenter
[0070] A 20L stirring fermenter (Nanjing Hualong Company) was used, and feeding was carried out according to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com