Solid oxide battery based on LSCF anode, preparation method of solid oxide battery and application of solid oxide battery in preparation of ethylene and ethane through oxidative coupling of methane
A solid oxide, LSCF-SDC technology, applied in the field of comfort and electrochemistry, can solve the problems of limited yield of C2 products, and achieve the effects of good thermoelectric reaction stability, high oxygen transport performance, and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
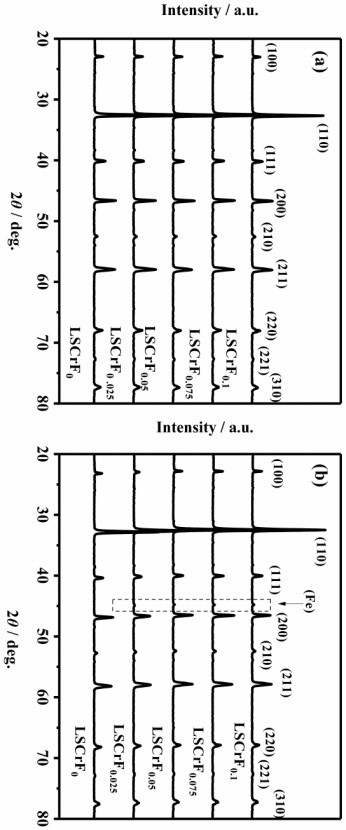
[0055] A series of ABOs 3 type perovskite material La 0.75 Sr 0.25 Cr 0.5 Fe 0.5+x O 3-δ (LSCrF x , x=0, LSCrF 0 ;x=0.025, LSCrF 0.025 ;x=0.05, LSCrF 0.05 ;x=0.075, LSCrF 0 .075 ;x=0.1, LSCrF 0.1 ) materials were synthesized by the liquid phase combustion method, and the LSCF in the oxidized state and the reduced state were x The powder was analyzed by XRD test. Figure 2(a) is the diffraction pattern of the oxidation state of the material, compared with the standard card PDF#01-075-0441, it can be seen that LSCrF x It is a pure perovskite phase, indicating a cubic structure with a space group of Pm-3m. figure 2 (b) for the material at 5 wt % H 2 The XRD diffraction pattern after pretreatment at 850 °C for 20 hours in an atmosphere of / 95wt% Ar shows that a metallic iron peak appears at 45.1 ° (PDF#96-230-0201). This clearly shows that after H 2 After / Ar pretreatment, the excess metallic iron at the B site can be converted from La 0.75 Sr 0.25 Cr 0.5 Fe 0.5...
Embodiment 2
[0057] (a) Zr 0.8 Y 0.2 O 2-δ The (YSZ) powder is pressed into a disc using a tablet press, and then placed in a muffle furnace for heat treatment at a temperature of 1500-1550 °C for 10-15 hours to obtain a dense solid ceramic electrolyte support;
[0058] (b) The different series of LSCrF x Powder and Ce 0.8 Sm 0.2 O 2-δ (SDC) was mixed at a ratio of 65:35, and then 10% by mass of ethyl cellulose and 5% of starch were added for uniform mixing. Finally, the mixed powder is uniformly ground, and 1-10 mL of turpentine is added dropwise during the grinding process, and the LSCrF required for the experiment is obtained by grinding for 3-5 hours. x -SDC composite electrode slurry, in which the LSCrFx powder is La 0.75 Sr 0.25 Cr 0.5 Fe 0.5+xO 3-δ (LSCrF x , x=0, LSCrF 0 ;x=0.025, LSCrF 0.025 ;x=0.05, LSCrF 0.05 ;x=0.075, LSCrF 0 .075 ;x=0.1, LSCrF 0.1 )
[0059] (c) The cathode electrode paste LSCrF-SDC was uniformly printed on one side of the electrolyte suppo...
Embodiment 3
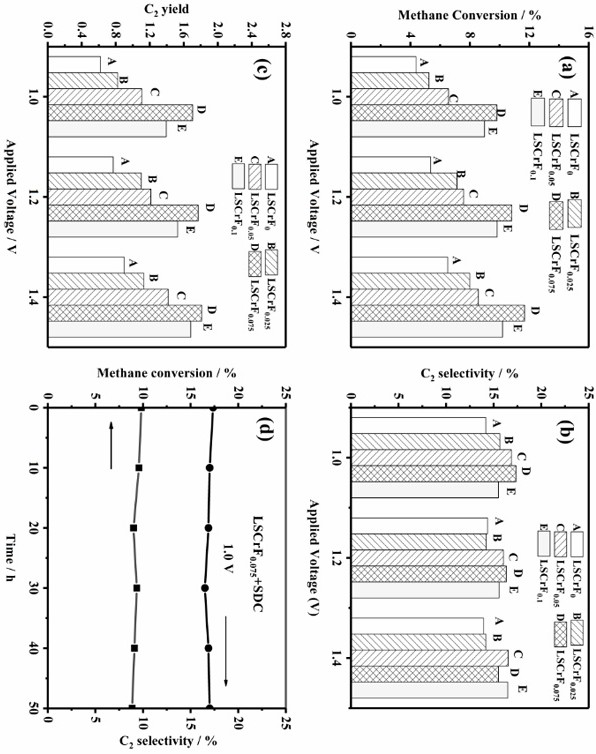
[0064] image 3 for different series of La 0.75 Sr 0.25 Cr 0.5 Fe 0.5+x O 3-δ Analysis results of anodic methane oxidation to ethylene and ethane. Supply 100 % concentration of CH to the anode 4 , and apply a voltage of 1.0-1.4 V while analyzing the products in the anode off-gas stream using on-line gas chromatography. As can be seen from Fig. 3(a) and Fig. 3(b), when the applied potential increases, the methane conversion also increases. When 1.4 V is applied, LSCrF 0.075 The methane conversion reached 11.7 % and the selectivity (C2) was found to be 15.5 %. Meanwhile, H was detected during the reaction 2 O and CO 2 The formation of , which is unavoidable during the reaction. from image 3 (c) It can be seen that as the applied potential increases, the LSCF 0.075 The yield of C2 product reaches a maximum of 1.8% at 1.4 V. with LSCrF without metal-oxide interface (no in situ precipitated Fe) 0 Compared to LSCrF 0.075 The constructed metal-oxide interface incr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com