Nifedipine composition and preparation method thereof
A technology for nifedipine and nifedipine, which is applied in directions such as drug combinations, pharmaceutical formulations, and non-active ingredients medical preparations, can solve problems such as increasing economic costs, reducing medication compliance, and increasing coating processes, thereby improving the The effect of stability, maintaining drug content and stable release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
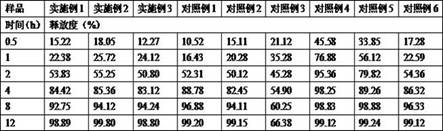
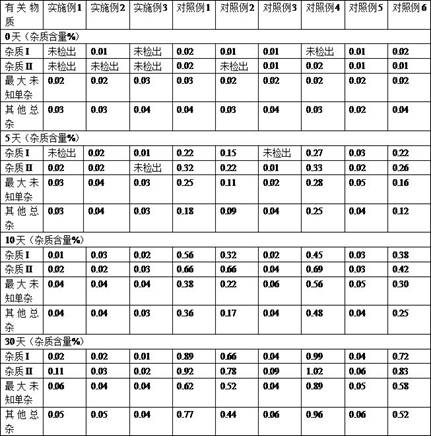
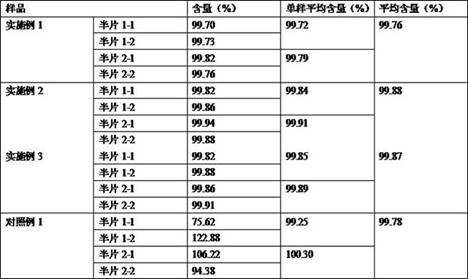
Examples
Embodiment 1
[0021] Example 1. Prescription:
[0022] Material name Dosage share Nifedipine 20g 20 servings microcrystalline cellulose 118g 118 servings pregelatinized starch 45g 45 servings alginate 10g 10 servings sodium bicarbonate 5g 5 servings Sodium stearate 2g 2 servings
[0023] Preparation:
[0024] Step 1: Adhesive preparation: Weigh 10g of kainate and add it into purified water at 75°C to prepare a solution with a concentration of 8%, add 5g of sodium bicarbonate, and measure pH=7.02. Then, 20 g of nifedipine was added, and the mixture was homogenized by a laboratory high-shear dispersing homogenizer emulsifier with a rotating speed of 25,000 rpm.
[0025] Step 2: Dry mixing: Weigh 118 g of microcrystalline cellulose and 45 g of pre-interleaved starch, add them to a wet granulator, set a stirring speed of 300 rpm and a chopping speed of 600 rpm, and mix for 5 minutes.
[0026] Step 3: Granulation: The mixed solut...
Embodiment 2
[0032] Material name Dosage share Nifedipine 20g 20 servings microcrystalline cellulose 130g 130 servings pregelatinized starch 35g 35 servings alginate 6g 6 servings sodium bicarbonate 8g 8 servings Sodium stearate 1g 1 serving
[0033] Preparation method: 1000 tablets were prepared with reference to the preparation method in Example 1, the difference was that 8% karate solution was prepared, 8 g of sodium bicarbonate was added, pH=7.47 was measured, and the solution was kept at 38°C, and the recipe quantity was referred to the dosage of Example 2.
Embodiment 3
[0035] Material name Dosage share Nifedipine 20g 20 servings microcrystalline cellulose 104g 104 servings pregelatinized starch 55g 55 servings alginate 14g 14 servings sodium bicarbonate 4g 4 parts Sodium stearate 3g 3 copies
[0036] Preparation method: prepare 1000 pieces with reference to the preparation method of Example 1, the difference lies in the configuration and configuration of 8% kelp solution, add 4g of sodium bicarbonate, measure pH=6.52, the solution is kept at 42°C, and the recipe quantity refers to the dosage of Example 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com