Pharmaceutical composition for treating premature ovarian failure as well as application and preparation method thereof
A premature ovarian failure and composition technology, which is applied in the field of pharmaceutical compositions for the treatment of premature ovarian failure, can solve the problems of not being able to meet the reproductive needs of patients, delaying treatment time, and high costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] In addition, the application also provides a preparation method of a pharmaceutical composition for treating premature ovarian failure, comprising:
[0081] Get the traditional Chinese medicine Astragalus, and extract the total astragalus saponins, astragaloside I, astragaloside II, astragaloside III and astragaloside IV in the pharmaceutical composition for treating premature ovarian failure as described in any one of the foregoing according to the natural product extraction method; Get one or more of the combinations to obtain the pharmaceutical composition for treating premature ovarian failure;
[0082] Among them, the natural product extraction method can be decoction, reflux extraction, ultrasonic extraction, microwave extraction, enzyme extraction, supercritical extraction, flash extraction, high-speed countercurrent chromatography and high performance liquid chromatography. one or more combinations.
[0083] As mentioned above, the traditional Chinese medicine ...
Embodiment 1
[0101] Example 1: Confirmation of components of monomers and extracts
[0102] Astragaloside total saponins, astragaloside I, astragaloside II, astragaloside III and astragaloside IV were separated and purified, and the above components were confirmed.
[0103] Specific methods and results:
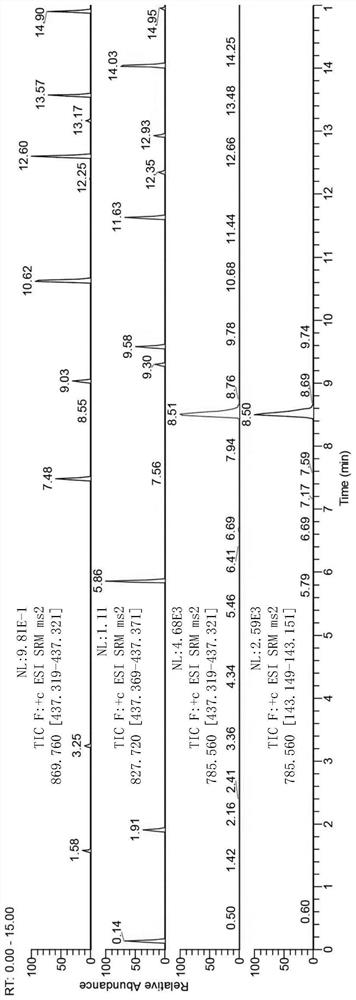
[0104] In this example, the total saponin extract of Astragalus, as well as Astragaloside I, Astragaloside II, Astragaloside III and Astragaloside IV were tested separately by high-resolution LC / MS to confirm their components.
[0105] Test Conditions:
[0106] Astragalus saponins I, II, III, IV and total astragalus saponins were respectively dissolved in 50% acetonitrile with a final concentration of 5 ppm. Using UPLCBEH C18 column (100mm×2.1mm, 1.7μm), 0.1% aqueous formic acid and acetonitrile were used as mobile phase A and mobile phase B, respectively, gradient elution (0-2.5min, 95%A→70%A; 2.5 -4.5min, 70%A→65%A; 4.5-6min, 65%A; 6-7min, 65%A→60%A; 7-9min, 60%A→40%A; 9-14min, 40% ...
Embodiment 2-4
[0118] Experimental Materials:
[0119] 1. Sample to be tested: total Astragalus saponins, Astragalus saponins I, Astragalus saponins II, Astragalus saponins III and Astragalus saponins IV.
[0120]2. Reagents and samples: pregnant horse serum gonadotropin PMSG, prospec hot-272; DMEM-F12 phenol red-free medium, Thermo; fetal bovine serum, Gibco; Accumax TM solution, Sigma; Hanks buffer, Thermo; CellTiter-Glo Luminescent Cell Viability Assay, Promega; Doxorubicin, Sigma; Mouse Follicle Stimulating Hormone (FSH) ELISA Kit, bio-swamp; Mouse Estradiol (E2) ELISA kit, bio-swamp; 4% paraformaldehyde tissue fixative, bio-swamp; hematoxylin-eosin staining solution, Baso; 1% hydrochloric acid alcohol, Baso.
[0121] 3. Instruments and equipment: cell incubator, Thermo; inverted microscope, ZEISS; inverted dissecting microscope, ZEISS; small cryogenic centrifuge, Eppendorf; cell counter, Countess II; multi-function microplate detector, BioTek.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com