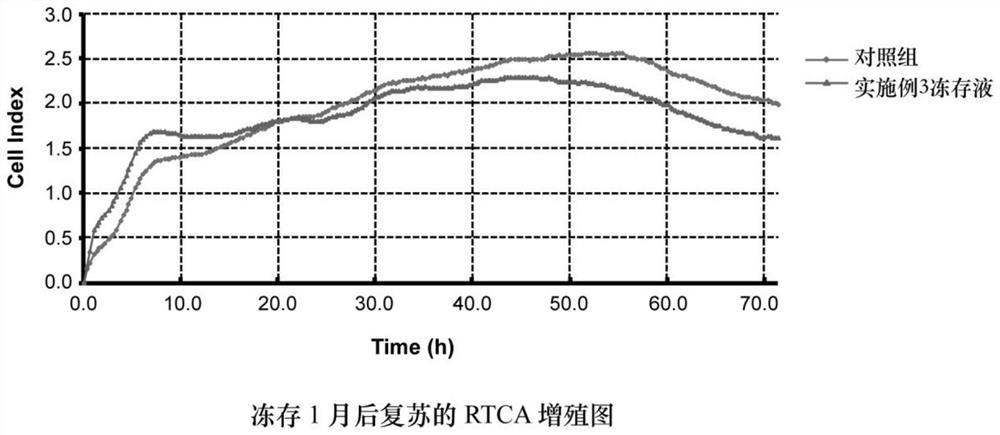
Cryopreservation liquid and cryopreservation method for long-term preservation of stem cells and stem cell products
A technology for long-term preservation and cryopreservation, which is applied in the preservation, application, and animal husbandry of human or animal bodies, and can solve the problems of self-renewal and uncertain factors in the differentiation process of cultured stem cells, and achieves a method that is beneficial to long-term preservation and preparation Simple, effective in reducing the formation of ice crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A cryopreservation solution for long-term preservation of stem cells and their products, including the following raw materials by weight: 10 parts of low-molecular-weight dextran, 2 parts of hydroxyethyl starch, 15 parts of chitosan, 3 parts of glycerol, and 3 parts of dimethyl sulfoxide 20 parts of glucose injection, 10 parts of compound amino acid injection and 20 parts of compound electrolyte injection.
[0029] The compound amino acid injection contains 6 g of L-leucine, 4 g of L-lysine acetate, 7 g of glycine, 3 g of methionine, 1 g of tyrosine, 2 g of glutamic acid, 2 g of threonine, and benzene per 1000 mL. 1 g of alanine, 1 g of aspartic acid, 1 g of cysteine, 1 g of serine, and 0.2 g of sodium bisulfite.
[0030] Compound electrolyte injection contains 3 g of calcium chloride dihydrate, 6 g of sodium chloride, 1 g of sodium gluconate, 1 g of sodium bicarbonate, 0.5 g of potassium chloride and 0.5 g of magnesium chloride per 1000 mL.
[0031] The glucose inject...
Embodiment 2
[0037] A cryopreservation solution for long-term preservation of stem cells and their products, including the following raw materials by weight: 15 parts of low molecular weight dextran, 8 parts of hydroxyethyl starch, 10 parts of chitosan, 6 parts of glycerol, 6 parts of dimethyl sulfoxide 30 parts of glucose injection, 15 parts of compound amino acid injection and 30 parts of compound electrolyte injection.
[0038] The compound amino acid injection contains 9 g of L-leucine, 8 g of L-lysine acetate, 9 g of glycine, 5 g of methionine, 3 g of tyrosine, 4 g of glutamic acid, 4 g of threonine, and benzene per 1000 mL. Alanine 3g, aspartic acid 3g, cysteine 3g, serine 3g and sodium bisulfite 0.4g.
[0039] Compound electrolyte injection contains 6 g of calcium chloride dihydrate, 9 g of sodium chloride, 3 g of sodium gluconate, 3 g of sodium bicarbonate, 0.8 g of potassium chloride and 0.8 g of magnesium chloride per 1000 mL.
[0040] The glucose injection contains 80 g of gl...
Embodiment 3
[0046] A cryopreservation solution for long-term preservation of stem cells and their products, including the following raw materials by weight: 12 parts of low molecular weight dextran, 6 parts of hydroxyethyl starch, 14 parts of chitosan, 4 parts of glycerol, 4 parts of dimethyl sulfoxide 16 parts of glucose injection, 13 parts of compound amino acid injection and 25 parts of compound electrolyte injection.
[0047] The compound amino acid injection contains 8 g of L-leucine, 5 g of L-lysine acetate, 8 g of glycine, 4 g of methionine, 2 g of tyrosine, 3 g of glutamic acid, 3 g of threonine, and benzene per 1000 mL. Alanine 2g, aspartic acid 2g, cysteine 2g, serine 2g and sodium bisulfite 0.3g.
[0048] Compound electrolyte injection contains 5 g of calcium chloride dihydrate, 8 g of sodium chloride, 2 g of sodium gluconate, 2 g of sodium bicarbonate, 0.6 g of potassium chloride and 0.6 g of magnesium chloride per 1000 mL.
[0049] The glucose injection contains 60 g of gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com