Tert-butyloxycarboryl protected acid-sensitive epoxy monomer as well as synthesis method and application thereof
A technology of tert-butoxycarbonyl and epoxy monomers, which is applied in the field of acid-sensitive epoxy monomers protected by tert-butoxycarbonyl and their synthesis, can solve the problems of lack of functionality, lack of reactive functional groups and the like, Achieve versatility, high added value, and low resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
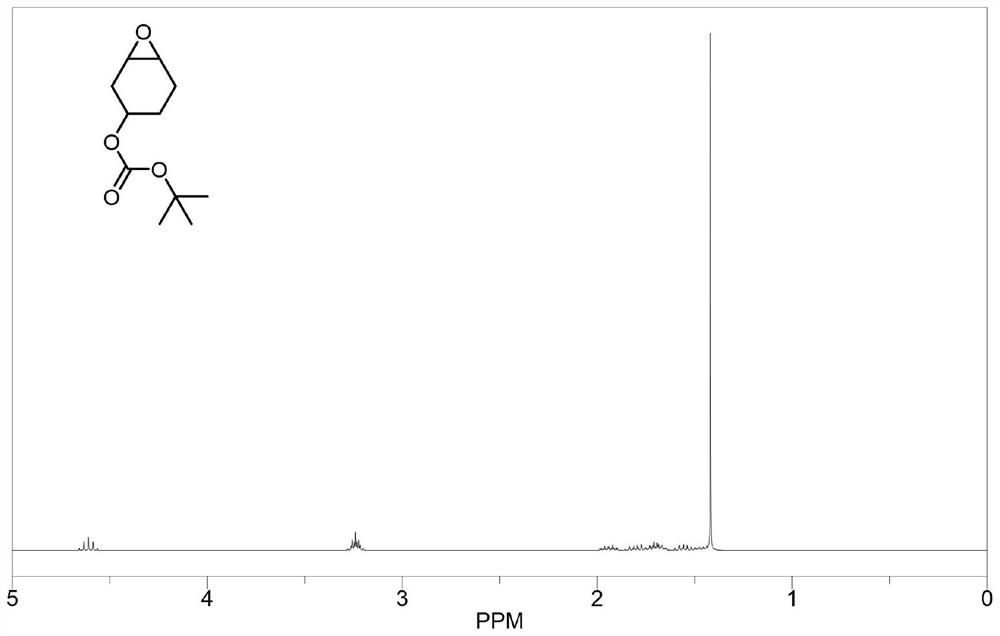
[0076] The structure and synthesis route of epoxy monomer A are as follows:
[0077]
[0078] Under nitrogen atmosphere, 68.25 g (1 molar equivalent) of tert-butoxyformyl chloride was added dropwise to 49 g of 1-hydroxy-3-cyclohexene, and 43.5 g (1.1 molar equivalent) of pyridine was added dropwise. After 1 h of reaction at room temperature, 200 ml of dichloromethane and 200 ml of deionized water were added to the reaction solution, the product was extracted into the dichloromethane phase, and the organic phase was washed three times with deionized water, anhydrous Na 2 SO 4 After drying, dichloromethane was removed by rotary evaporation, and then distilled under reduced pressure to obtain raw material a.
[0079] Dissolve raw material a in 1 L of dichloromethane, add 110 g (purity of 85 wt%, 1.2 molar equivalent) m-chloroperoxybenzoic acid (m-CPBA), react at room temperature for 12 h, add saturated NaSO 3 Solution until starch potassium iodide test paper does not turn blue...
Embodiment 2
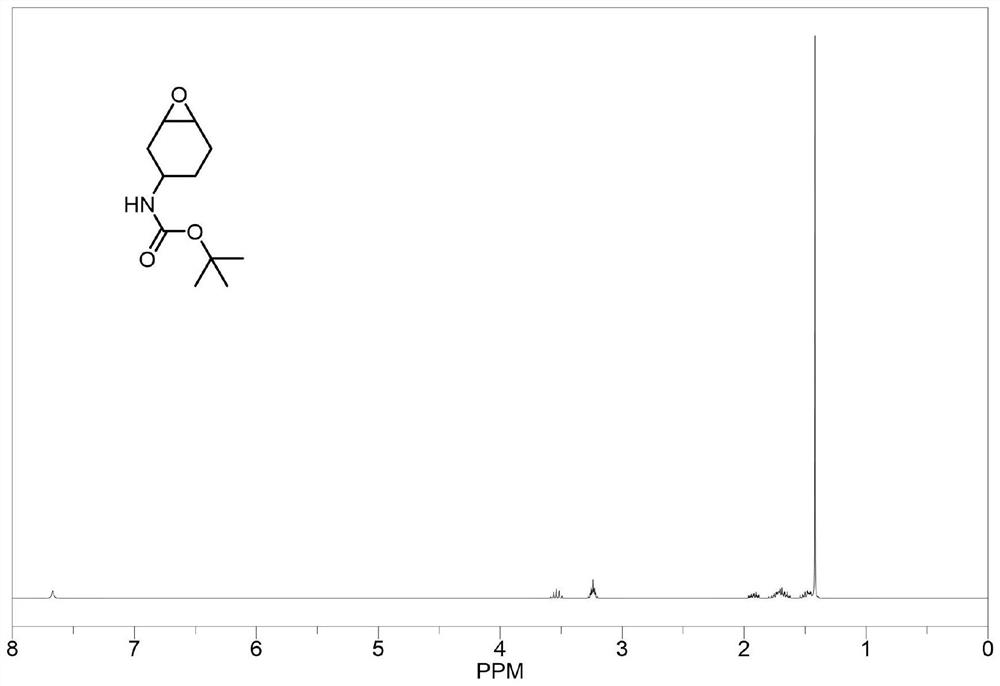
[0081] The structure and synthesis route of epoxy monomer B are as follows:
[0082]
[0083] At 0°C, 48.5 g of 3-aminocyclohexene was added to a mixed solution of 500 ml of dioxane and 300 ml (2M aqueous solution, 1.2 molar equivalent) sodium hydroxide solution, and 130 ml ( 1.1 molar equivalents) di-tert-butyl dicarbonate. After the reaction was stirred at room temperature for 5 h, 300 ml of deionized water was added, and the pH value was adjusted to 3 with 1.5 N hydrochloric acid. The reaction solution was extracted with ethyl acetate (3×200ml), washed with saturated NaCl solution 3 times, and anhydrous Na 2 SO 4 After drying, ethyl acetate was removed by rotary evaporation and then distilled under reduced pressure to obtain raw material b.
[0084] Dissolve raw material b in 1 L of dichloromethane, add 97.5 g (purity of 85 wt%, 1.2 molar equivalent) m-chloroperoxybenzoic acid (m-CPBA), and react at room temperature for 12 h, then add saturated NaSO 3 Solution until ...
Embodiment 3
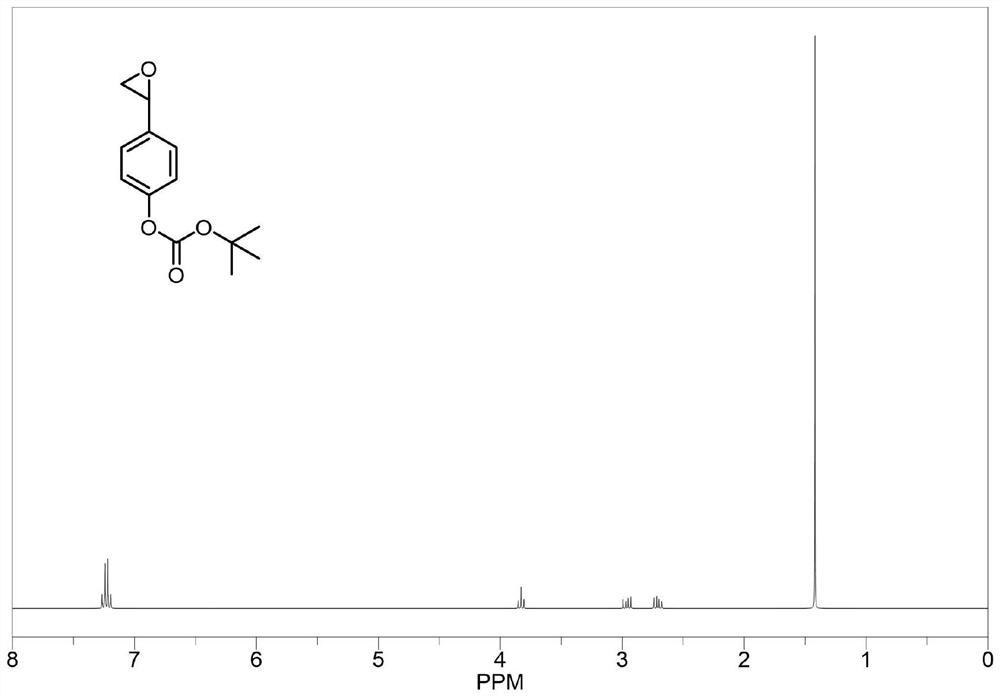
[0086] The structure and synthesis route of epoxy monomer C are as follows:
[0087]
[0088] The epoxy monomer C can be obtained by the same synthesis method as in Example 1, except that the 1-hydroxy-3-cyclohexene in the carbonation reaction is changed to 4-vinylphenol. by as image 3 As can be seen from the shown proton nuclear magnetic resonance spectrum, epoxy monomer C was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com