Method for synthesizing 2-aryl-1-cyclohexanol based on continuous flow reaction technology
A cyclohexanol reaction technology, applied in the field of preparation of 2-aryl-1-cyclohexanol based on continuous flow reaction technology, can solve the problem of high risk of tert-butyllithium/butyllithium, high requirements for reaction operation, and difficulty in scaling up Production and other issues, to achieve the effect of reducing dangerous operation coefficient, good heat and mass transfer, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
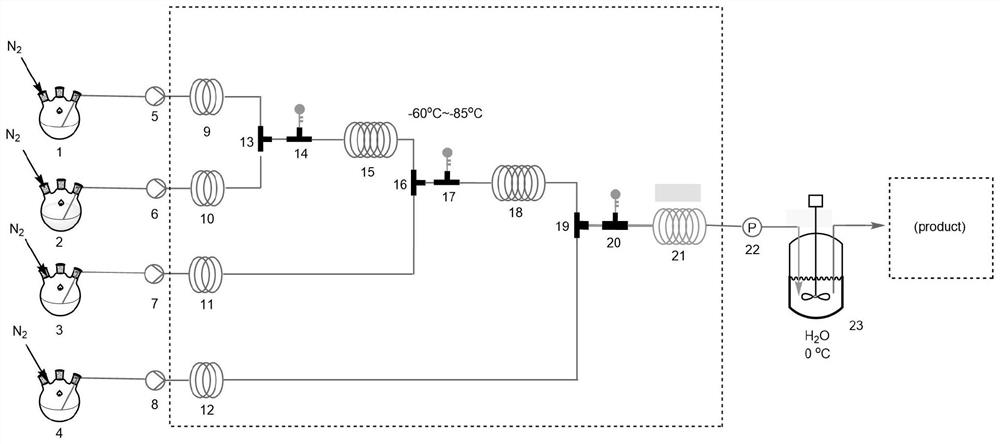
[0051] like figure 1 As shown, the THF solution of bromobenzene is packed in there-necked flask 1, nBuLi is packed in there-necked flask 2, the THF solution of cyclohexene oxide is packed in there-necked flask 3, and the toluene solution of boron trifluoride ether is packed into there-necked flask 3 In 4, the temperature control device is set to -60 ℃, and the THF solution of bromobenzene and nBuLi are pumped in by continuous flow experimental pumps 5 and 6 respectively, so that it is carried out lithium halide exchange 6min in reaction tube 15; The THF solution of intermediate 2 and cyclohexene oxide was subjected to nucleophilic substitution in reaction tube 18 for 4 min; finally, the intermediate 3 obtained in the second step and the toluene solution of boron trifluoride ether were subjected to ring opening in reaction tube 21 , the retention time was 7min; then the reactant was passed into 23 (with 1 / 3 of the ice-water mixture) for quenching, and then the reaction solution...
Embodiment 2
[0054] The concrete synthesis process is the same as the embodiment of the present invention 1, except that bromobenzene is replaced with 2-methylbromobenzene, and the temperature control device is set to -65°C; the purity of the obtained product is 87.2%;
[0055] 1 HNMR (400MHz, CDC1 3 )δ7.27-7.23(m,1H),7.22-7.14(m,2H),7.13-7.08(m,1H),3.83-3.71(m,1H),2.83-2.68(m,1H),2.36( s,3H),2.18-2.07(m,1H),1.94-1.70(m,3H),1.69-1.59(m,1H),1.51-1.27(m,4H).
Embodiment 3
[0057] The specific synthesis process is the same as the embodiment of the present invention 2, except that 2-methyl bromobenzene is replaced with 3-methyl bromobenzene; the purity of the obtained product is 85.9%;
[0058] 1 HNMR(400MHZ,CDC1 3 )δ7.26-7.18(m,1H),7.09-7.01(m,3H),3.70--3.58(m,1H),2.43-2.35(m,1H),2.34(s,3h),2.14-2.06 (m,1h),1.88-1.71(m,3H),1.68-1.60(m,1H),1.56-1.28(m,4h).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com