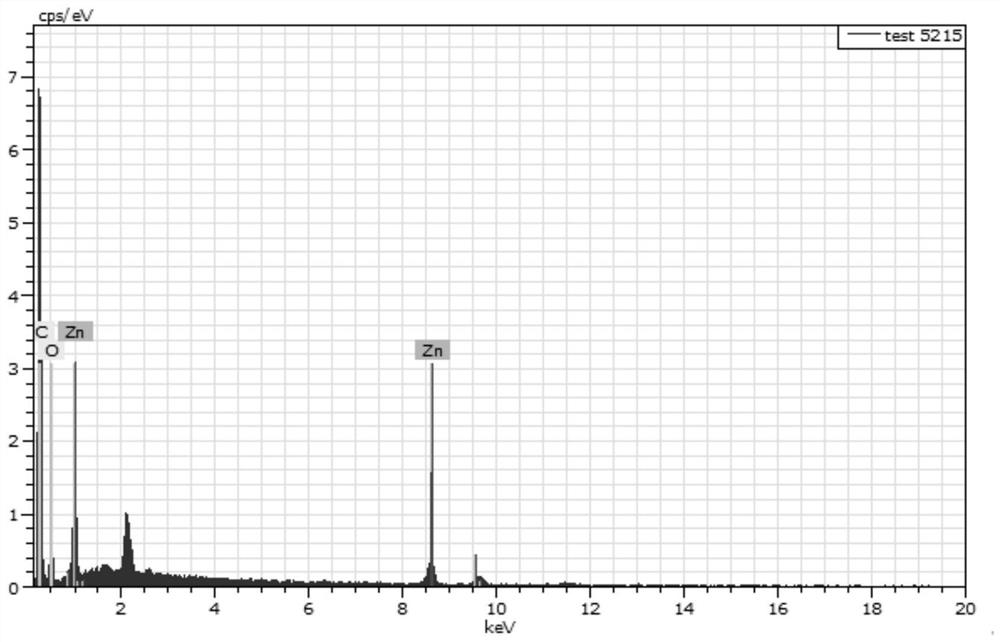
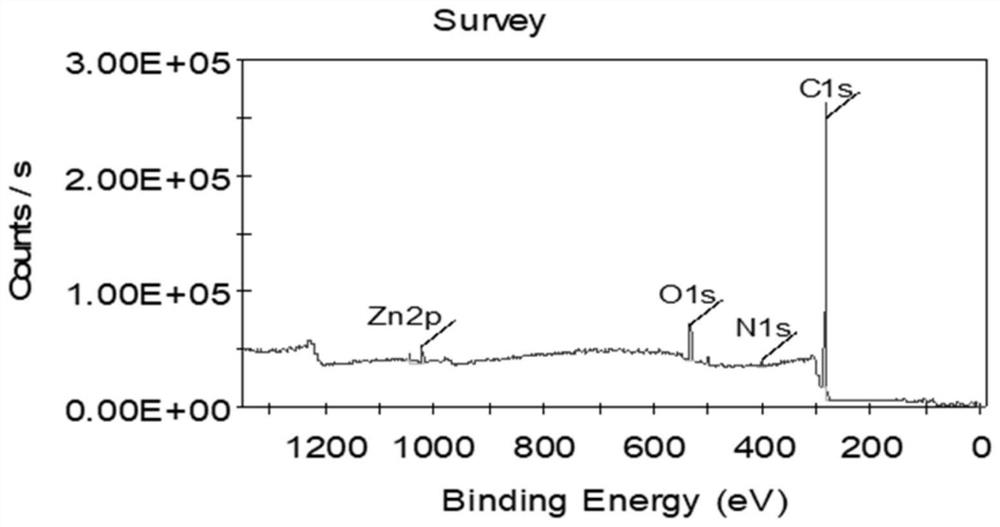
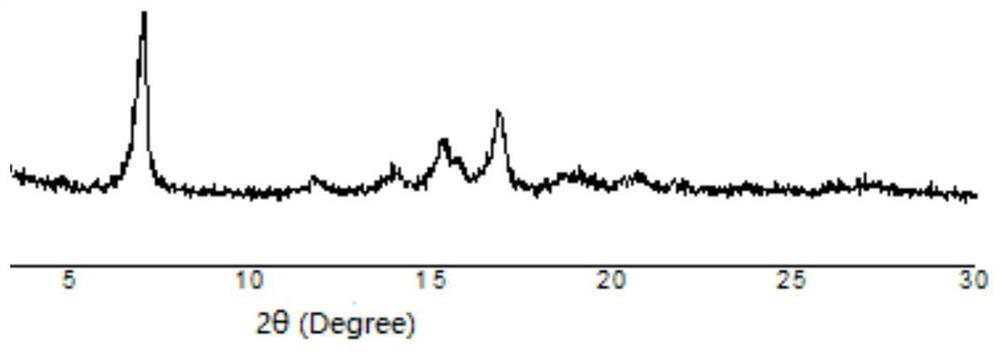
Efficient catalysis method for Knoevenagel condensation reaction
A condensation reaction, high-efficiency technology, used in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, preparation of organic compounds, etc. problem, to achieve the effect of efficient catalytic condensation reaction and simple material recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthetic method of tetrahydroxytetraaldehyde tetraphenylene is as follows:
[0034]
[0035] In a 500mL three-necked flask, firstly add the weighed zinc powder (35.9g, 553.31mmol) and a stirring magnet, sort out the brine sealing system, set up a reflux condensing device, and then repeat the vacuum evacuation and fill with nitrogen three times. Add 190 mL of freshly purified anhydrous tetrahydrofuran (THF) with a standard syringe, cool it to 0°C under an ice-water bath, and then slowly add a quantitative amount of titanium tetrachloride (30.0 mL, 280.08 mmol) with a syringe of suitable size, inject After the end, continue to stir in an ice-water bath for about 15 minutes, then heat and reflux for 3.0h, after that, stop heating and let the reaction system cool to room temperature, then slowly add quantitative 4,4-dihydroxydiphenyl using a constant pressure dropping funnel A solution of methyl ketone (9.1 g, 42.01 mmol) dissolved in 100 mL of anhydrous THF (tetrah...
Embodiment 2
[0037] The synthesis method of the catalyst for the Knoevenagel condensation reaction is as follows:
[0038] Accurately weigh 5 mg of tetrahydroxytetraaldehyde tetraphenylethylene, 5 μL of ethylenediamine, 1.5 mL of 1,4 dioxane, Zn(OAC) 2 ·2H 2 O 20.8 mg, 0.2 mL of 3M aqueous glacial acetic acid. Put the weighed medicine into a prepared glass bottle that can withstand high temperature and low temperature at the same time, freeze, thaw, and reciprocate three times under the conditions of liquid nitrogen cooling and oil pump vacuuming. The tube was sealed with a flame gun, the sealed glass bottle was put into a beaker, and the beaker was put into an oven at 120° C. to react for 72 hours.
[0039] Three days later, take out the glass tube, open the seal, take out the solid insoluble matter, and slowly wash it on a separatory funnel with the large polar solvent tetrahydrofuran, DMF, and methanol. After that, the product was put into a vacuum oven at 60 °C for one day and one n...
Embodiment 3
[0043] An efficient catalytic method for Knoevenagel condensation reaction, using acetonitrile as a solvent, in the presence of the catalyst prepared in Example 2, the reaction temperature of aldehyde and malononitrile is 60°C, and the reaction time is 15 minutes.
[0044] 1. The best solvent for COFs to catalyze the Knoevenagel condensation reaction
[0045]Take four dry test tubes and number them ①②③④. Add COF-Zn (5mg), ultra-dry methanol (1mL) and p-chlorobenzaldehyde (20mg) to the test tube No. 1 in turn, and add COF-Zn (5mg) to the test tube No. , p-chlorobenzaldehyde (20mg), malononitrile (100mg) and 1,2 dichloromethane (1mL), 5mgCOF-Zn + p-chlorobenzaldehyde (20mg) + malononitrile (100mg) + acetonitrile were added to the test tube No. (0.1 mL), 5 mg of COF-Zn + p-chlorobenzaldehyde (20 mg) + malononitrile (100 mg) + 1,4 dioxane (0.1 mL) were added to the test tube No. 4 in turn. Put the four test tubes into a constant temperature magnetic stirrer with a temperature of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com