AMP phosphotransferase mutant, coding gene thereof and application of AMP phosphotransferase mutant in ATP synthesis
A phosphotransferase and mutant technology, applied in the field of genetic engineering, can solve the problems of difficult process control, many types of enzymes, low enzyme activity, etc., and achieve the effects of cost reduction, less impurities and pigments, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Obtaining method of AMP phosphotransferase mutant
[0043] Random mutation was introduced by random mutation PCR kit, and the difference in ATP synthesis ability between mutant and wild type was screened by HPLC method. Select forward mutant monoclonal strains, extract plasmids and send them for sequencing to obtain mutation points. A total of 4 single point mutations were obtained, see mutants 1-4. In the later stage, mutants 5-10 were constructed by introducing site-directed mutagenesis through primer design and tested for activity.
[0044] A total of ten strains with high vigor and high stability were obtained by the viability screening method under the same conditions, which were named as mutants 1-10, which contained mutations at positions 124 (A124L), 312 (G312M) and 393 (F393K). Body 9 (named AMP-PPTM enzyme) can increase about 80 times in ATP synthesis activity, about 20 times in temperature stability, and the most significant improvement in activity...
Embodiment 2
[0047] Example 2: Determination of AMP-PPTM enzyme properties
[0048] The plasmids containing AMP phosphotransferase and its mutant were respectively transformed into Escherichia coli BL231 (DE)) to obtain mutants, the strains were inoculated in LB medium, and the OD of the bacteria was treated. 600 When the value reached 0.6, the final concentration of 0.1 mM IPTG was added to induce the expression of AMP phosphotransferase and its mutants, overnight induction, and the induction condition was 20°C. The amount of cells collected horizontally in shake flasks was 5 g / L. The bacterial cells were crushed to prepare a crude enzyme solution.
[0049] According to the activity value of AMP-PPTM enzyme, high-quality strains were determined. Enzyme activity test 1mL reaction system: substrate adenosine (13.5mM / L), ATP disodium salt (5mM / L), magnesium ion (50mM / L) and sodium hexametaphosphate (50mM / L), pH7.0, The reaction was performed at 37°C for 30 min, and the amount of ATP gener...
Embodiment 3
[0050] Example 3: Determination of the detection method of each substance in the catalytic reaction
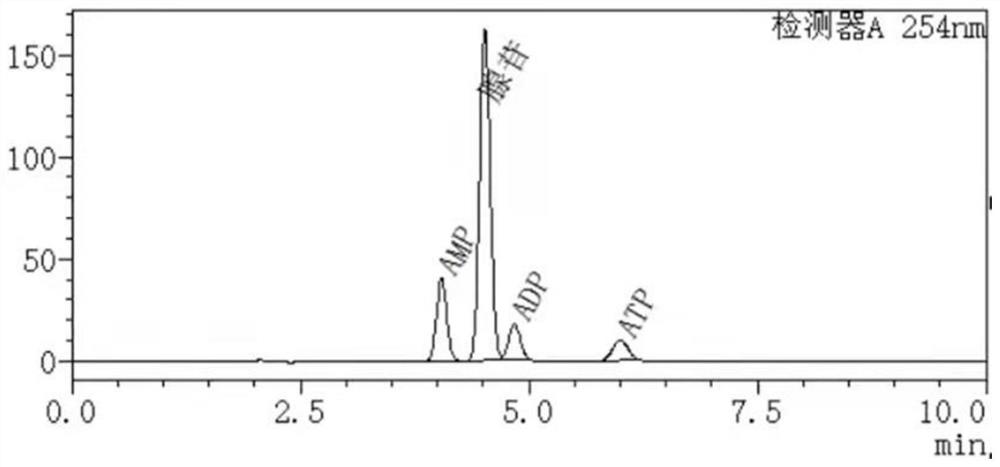
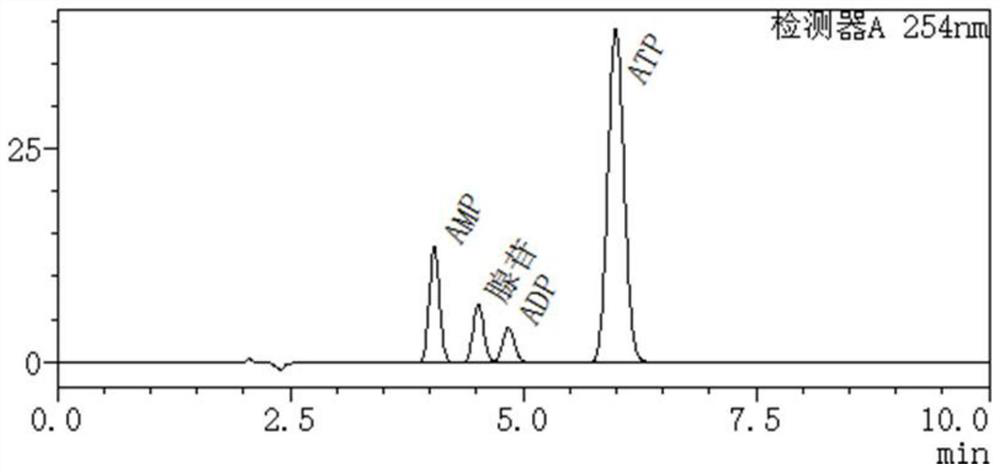
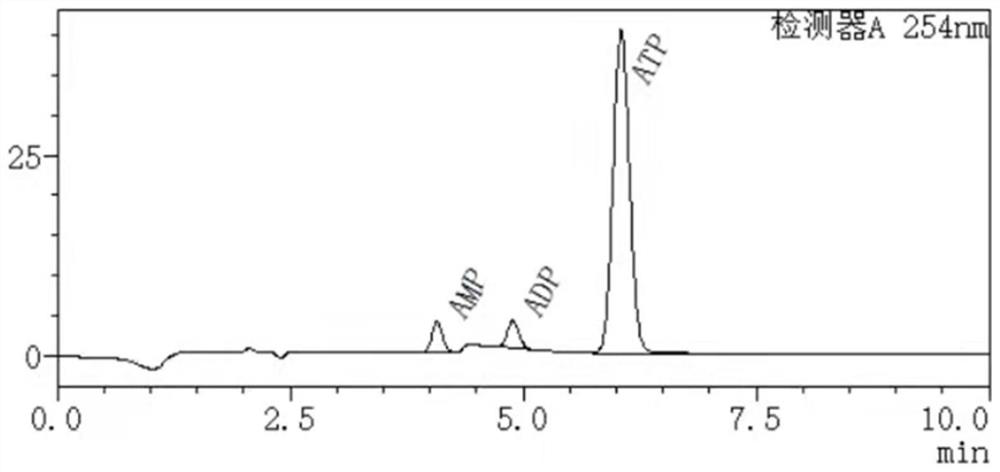
[0051] High performance liquid chromatography was used to detect the remaining substrate of the catalytic reaction and the formation of the product. Chromatographic conditions: C18 reversed-phase column, mobile phase phosphate buffered saline-methanol, flow rate 1.2 mL / min, column temperature 30 °C, wavelength 254 nm. Test the 100mL reaction system, react for 30min, and detect the conditions of each substance in the system, such as figure 1 : The ATP generation rate is 33%, and a little AMP and ADP are generated; the reaction is 2h, and the ATP generation is detected, such as figure 2 : The generation rate of ATP is 81%, followed by AMP; after 3 hours of reaction, the amount of ATP generated is detected, such as image 3 , the substrate was basically consumed completely, and the ATP generation rate was 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com