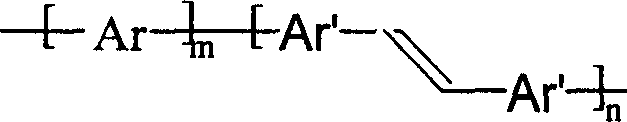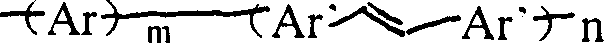Copolymer type conjugated polymer whose main chain contains double bond, its preparation method and application
A conjugated polymer and copolymerization technology, used in chemical instruments and methods, luminescent materials, etc., can solve problems such as difficulty in adapting to high-performance devices, difficulty in obtaining batch products, and complex synthesis work, and achieve enhanced dissolution and processing performance. The effect of simplified preparation steps and more flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 0.40 g of heptylcarbazole and 0.54 g of trans-1,2-stilbene were added to a 250-mL three-necked flask, 75 mL of freshly distilled chloroform was added, stirred to dissolve, 2.93 g of dry anhydrous ferric chloride was added, and 2.93 g of dry anhydrous ferric chloride was added. The reaction was carried out at room temperature for 24 hours under the protection of dry nitrogen. The reaction was stopped, the ferric chloride was removed by suction filtration on the funnel, the filter residue was washed several times with chloroform, and the filtrates were combined. The filtrate was poured into 120 mL of methanol, and after stirring for 2 hours, the product precipitated, and the product was collected, washed with methanol, and dried at 60°C to obtain 0.57 g of a brown powder. Intrinsic viscosity 0.3-10.0g·dL -1 . The infrared, ultraviolet and nuclear magnetic resonance spectra prove that it is a copolymer, and the fluorescence spectrum proves that its maximum emission inten...
Embodiment 2
[0022] 3.7 g of triphenylamine and 2.7 g of trans-1,2-stilbene were dissolved in 200 ml of chloroform to obtain a bright green solution. In addition, under the protection of drying, 300 ml of chloroform and 10 g of anhydrous ferric chloride were added to a 500-ml reaction flask, and the aforementioned triphenylamine and stilbene solutions were added dropwise, and the reaction was stopped by stirring at room temperature for 24 hours. After filtration, the filtrate was slightly concentrated to about 200 ml, and after cooling, about 500 ml of methanol was added, stirred for 1 hour, and suction filtered to obtain a black flake precipitate. It was dried at 60° C. to obtain 1.9 g of the product, which was identified as a copolymer by infrared, elemental analysis and nuclear magnetic resonance, and its emission peak was about 430 nanometers by fluorescence analysis, indicating that it was a blue light emitting material.
Embodiment 3
[0024] 0.32 g of 9,9-dihexylfluorene and 0.30 g of 1,2-(2,5-dithiophene)ethylene were dissolved in 30 ml of carbon tetrachloride and the solution was placed in a dropping funnel. The funnel is connected to the three-necked reaction flask, and the reaction flask is also provided with a condensing water pipe and an air conduit. Under the protection of dry nitrogen or argon, 0.35 g of anhydrous ferric chloride, 0.3 g of anhydrous copper dichloride and 20 ml of carbon tetrachloride were added to the reaction flask, and dihexyl fluorene and dithiophene were added dropwise with stirring. The ethylene mixture was stirred at room temperature for 30 hours. Stop stirring and filter, pour the filtrate into 150 ml of methanol, stir for 1 hour, filter, wash the filter residue with petroleum ether and methanol several times, and dry to obtain 0.29 g of the product. The product has good solubility in chloroform and tetrahydrofuran, and is proved to be a copolymer by elemental analysis, infr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com