Biological fluid film of recombined human keratinized cell growth factor-2 and its preparing process
A biological fluid, keratinocyte technology, applied in recombinant DNA technology, drug combination, pharmaceutical formulations and other directions, can solve the problems of restricting the wide use of solid dressing films, complex process, inconvenience, etc., and achieves improved industrialization value and simple extraction process. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
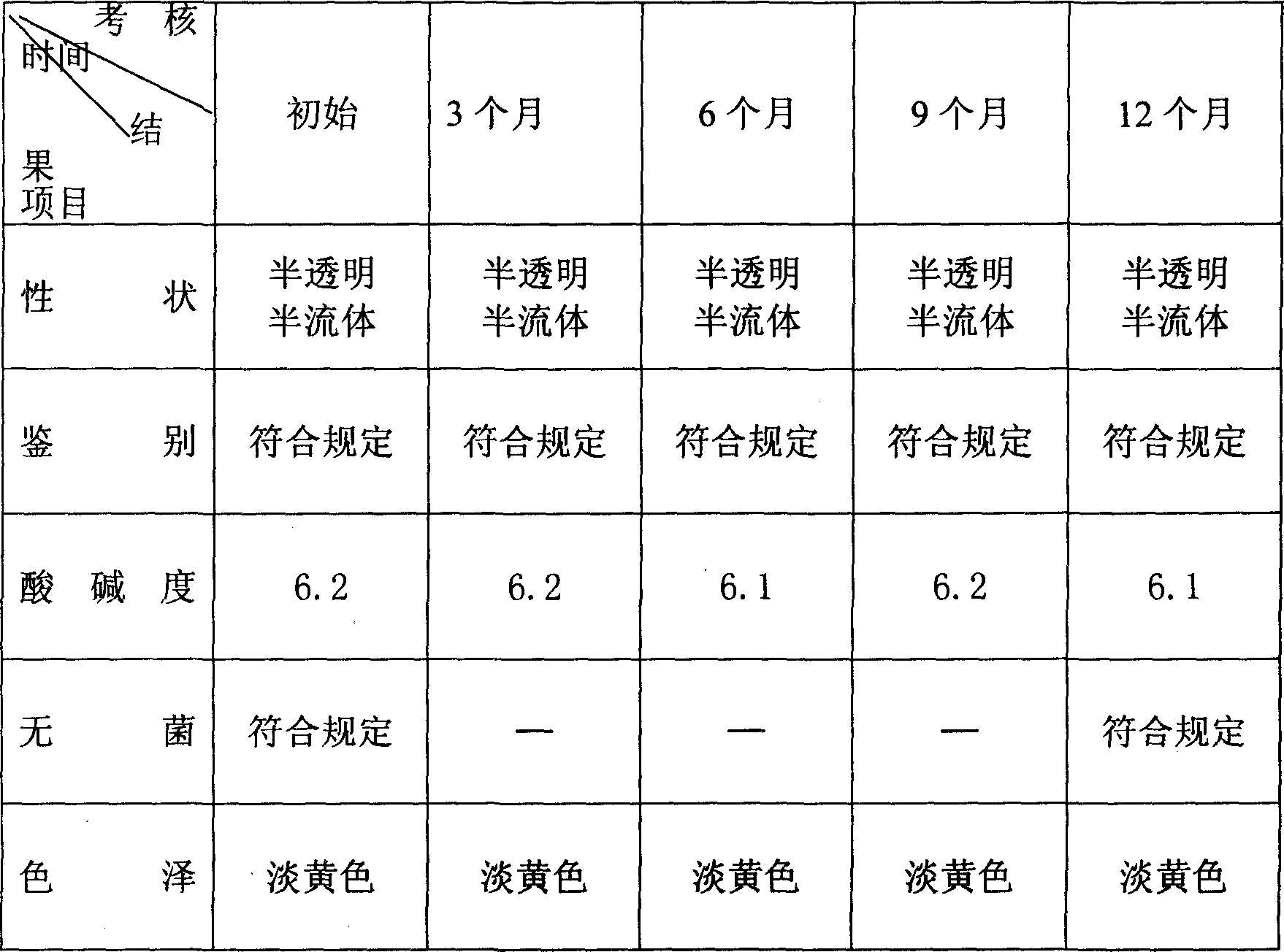
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of recombinant human keratinocyte growth factor-2 biological fluid membrane matrix:
[0051] Take by weighing 492 grams of chitosan dried at 80°C (final concentration is 1.6%) and put in a stirrer, add 25 kilograms of purified water and stir evenly, the stirring speed is 196r / min, slowly add 250ml acetic acid solution while stirring, the pH value For 3, the process takes about 12 hours. After the completion, add 150g of gelatin dissolved in 1500ml of water and 150ml of glycerin while it is hot, mix well, and then slowly adjust the pH value of the chitosan solution to 6.5 with 3700ml of 0.7% sodium bicarbonate solution. , the process takes 2-3 hours, then add 24.5 grams of polyvinylpyrrolidone, fully stir, filter, heat, remove air bubbles, sterilize, measure the pH value and viscosity of the chitosan solution the next day, and then potting and packaging in medical aluminum tubes. The viscosity measured with a rotary viscometer is 3570mpa.s. The...
Embodiment 2
[0062] Embodiment 2: Preparation of recombinant human keratinocyte growth factor-2 stock solution:
[0063] The expression plasmid is pLY4, and the host bacteria is Escherichia coli BL 21 (D3). According to the cDNA sequence of KGF-2 in the gene library, synthesize 20 complementary oligonucleotides, and introduce specific enzyme cutting sites EcoR I and BamH I at the 5' end and 3' end respectively, and adopt the conventional method of molecular cloning , first treated with T4 phage polynucleotide kinase at 37°C for 30 minutes. Phosphorylated oligonucleotide fragments were mixed in equimolar numbers, denatured at 94°C for 5 minutes, and immediately annealed at 65°C for 10 minutes, then added T4 ligase and reacted overnight at 16°C.
[0064] The pLY4 plasmid was digested with EcoR I and BamH I, and the large fragment was recovered. It was ligated with the synthetic rhKGF-2 cDNA fragment overnight, and directly transformed into E. coli host strain BL 21 (D3), and then screened f...
Embodiment 3
[0065] Embodiment 3: the preparation of rhKGF-2 biological fluid film
[0066] A new formulation of rhKGF-2-fluid film is prepared by matching the fluid dressing film with an appropriate concentration of recombinant human keratinocyte growth factor (rhKGF-2), and its formula is designed as follows:
[0067] Take 1 part of sterile 0.4% recombinant human keratinocyte growth factor-2 (rhKGF-2) aqueous solution, slowly add it to 19 parts of rhKGF-2 biological fluid membrane matrix, fully stir, filter, defoam, and fill in 80 ℃ baking 4h in the medical aluminum tube. The final concentration of rhKGF-2 in the fluid film was 200μg / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com