Lithium based phosphates for use in lithium ion batteries and method of preparation
A lithium-ion battery, lithium-ion technology, applied in lithium storage batteries, battery electrodes, secondary batteries, etc., can solve the problem of low electricity of alkaline transition metal oxides, and achieve easy commercial production, low cost, and comparable electricity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0060] The following describes the formation of Li 3 M’M” (PO 4 ) 3 Preferred steps for compounding active materials. Li 3 M’M” (PO 4 ) 3 The preparation method will use Li 3 V 2 (PO 4 ) 3 (Li 3 m 2 (PO 4 ) 3 ) formation to illustrate. The basic steps include making a lithium compound preferably lithium carbonate, a metal oxide preferably vanadium pentoxide and a phosphoric acid derivative preferably ammonium phosphate, ammonium phosphate NH 4 h 2 (PO 4 ) or (NH 4 ) 2 H(PO 4 ) to react. Each precursor material was purchased from a number of chemicals including Aldrich Chemical Company and Fluka. with approximately stoichiometric Li 2 co 3 , V 2 o 5 and (NH 4 )2 HPO 4 Preparation of Li from a mixture of 3 V 2 (PO 4 ) 3 . However, using a 5% excess of lithium (in the form of lithium carbonate) makes (Li 2 (O form) loss of lithium is minimized. The precursor materials were mixed carefully and then ground in a solution of methanol for about 30 minu...
Embodiment II
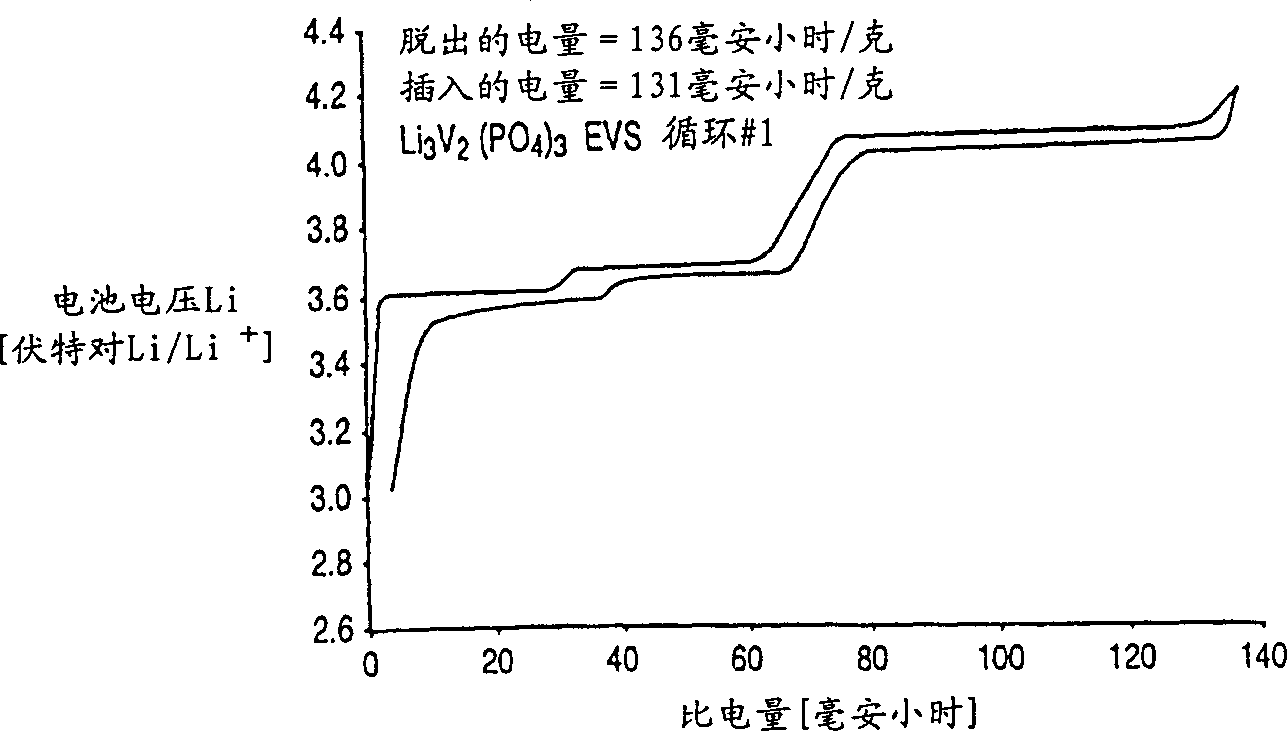
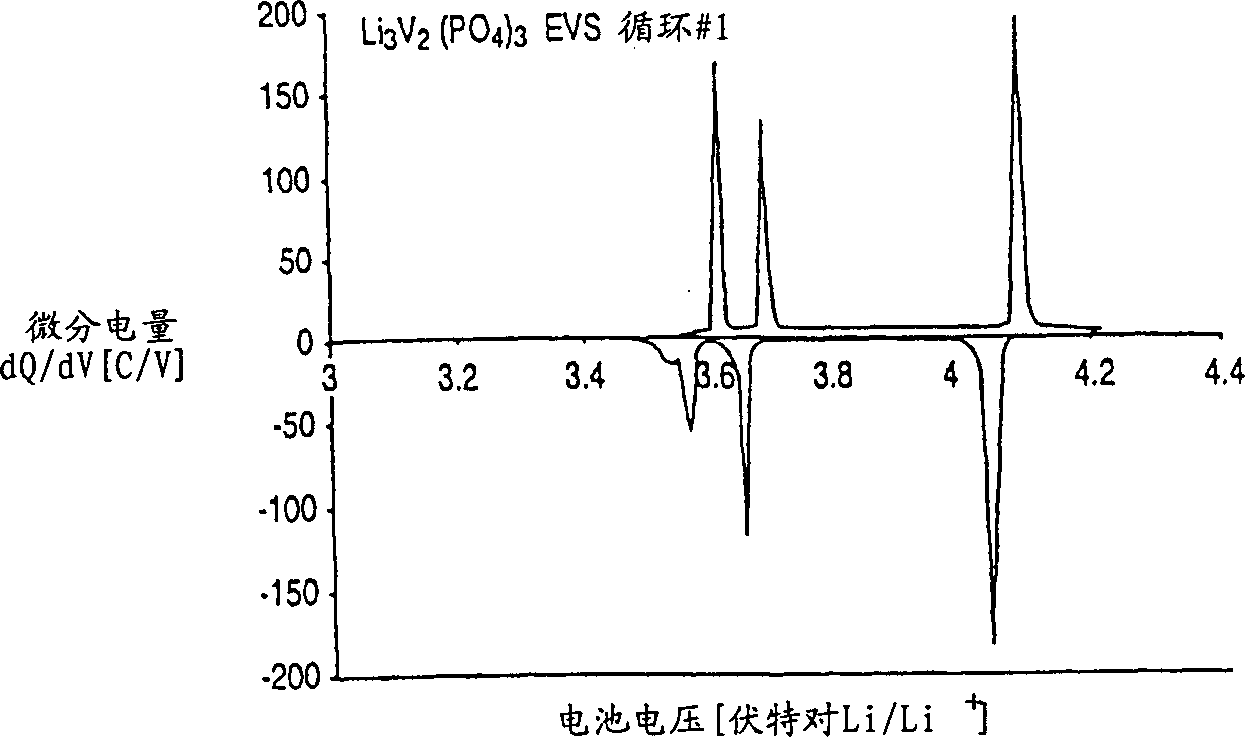
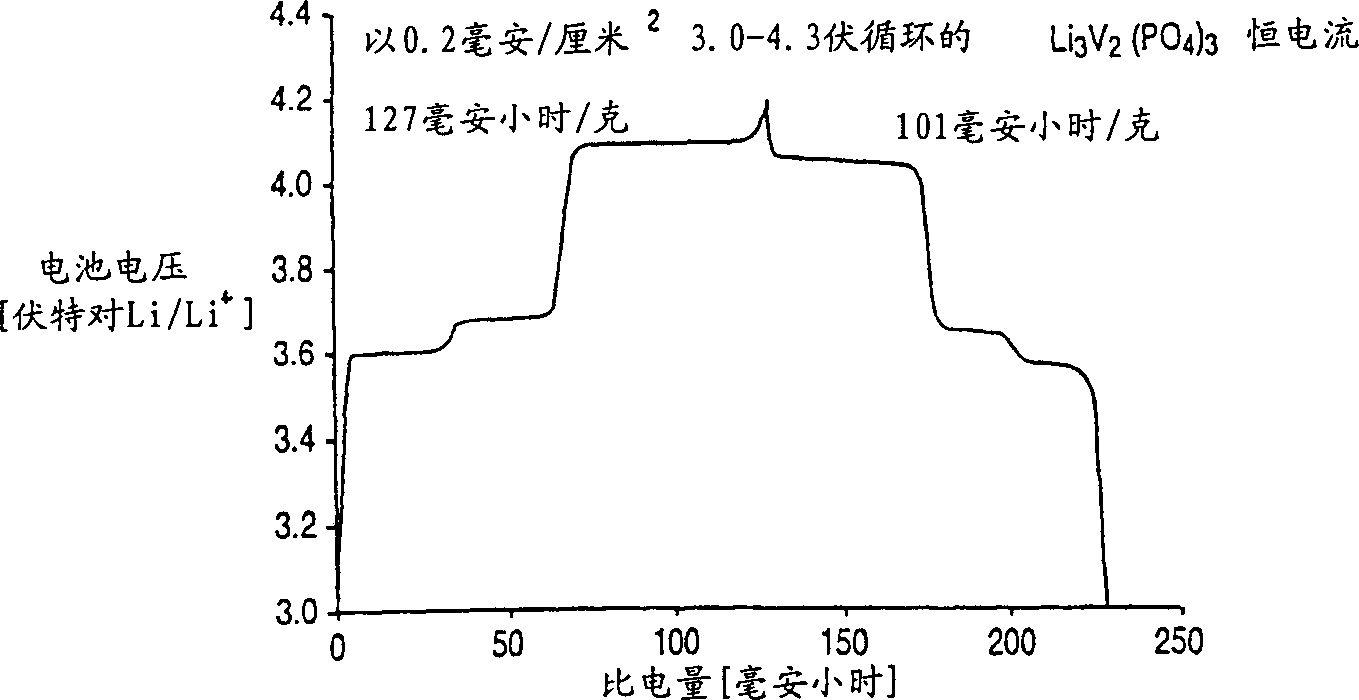
[0065] The Li prepared above was immediately tested in an electrochemical cell 3 V 2 (PO 4 ) 3 . A positive electrode was first prepared from this compound as described in the "Positive Electrode" section. The negative electrode is lithium metal. The electrolyte is a 2:1 weight ratio mixture of ethylene carbonate and dimethyl carbonate with 1 molar concentration of LiPF dissolved in it 6 . The battery cycles between about 3.0-4.3 volts and performs as figure 1 , 2 , 3, 4A and 4B.
[0066] figure 1 shows the voltage graph of the test cell, with the Li 3 M’M” (PO 4 ) 3 Based on the positive active material, a lithium metal counter electrode is used. figure 1 The data shown in are using the electrochemical voltage spectroscopy (EVS) technique. Electrochemical and kinetic data were recorded using the electrochemical voltage spectroscopy (EVS) technique. This technique is known in the art as described in J. Barker in Synth Met 28, D217 (1989), Synth Met 32, 43 (1969)...
Embodiment III
[0070] Embodiment III (hexagonal crystal Li 3 AlV(PO 4 ) 3 )
[0071] The following describes the preparation of Li 3 AlV(PO 4 ) 3 Methods. The basic steps include the reaction between lithium carbonate, aluminum hydroxide, vanadium oxide and ammonium phosphate according to the following equation:
[0072]
[0073] As described in Example 1, the precursor materials were first carefully ground and mixed. After mixing, the mixed powdered precursors were pressed into pellets, followed by heating at about 250° C. for about 6 hours under an argon atmosphere. The temperature was then increased to 600°C, also under argon, for 12 hours. Then, cool, grind and then granulate. Then, it was heated at a temperature of about 940° C. for about 15 hours under an argon atmosphere.
[0074] CuKα X-ray diffraction analysis was performed on the final product as described in Example 1. The unit cell parameters are shown in Table H.
[0075] Chemical analysis result and X-ray diagra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com