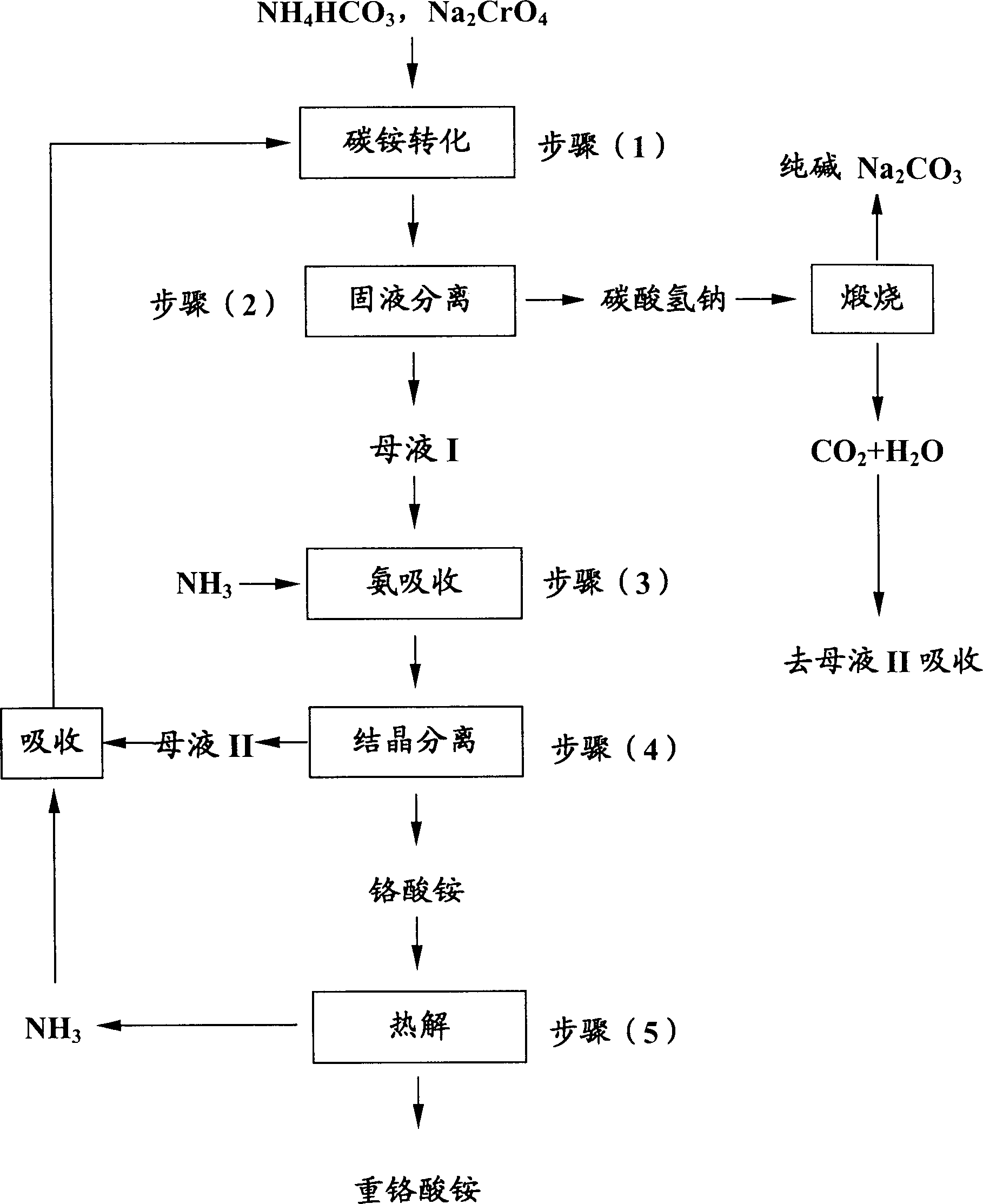
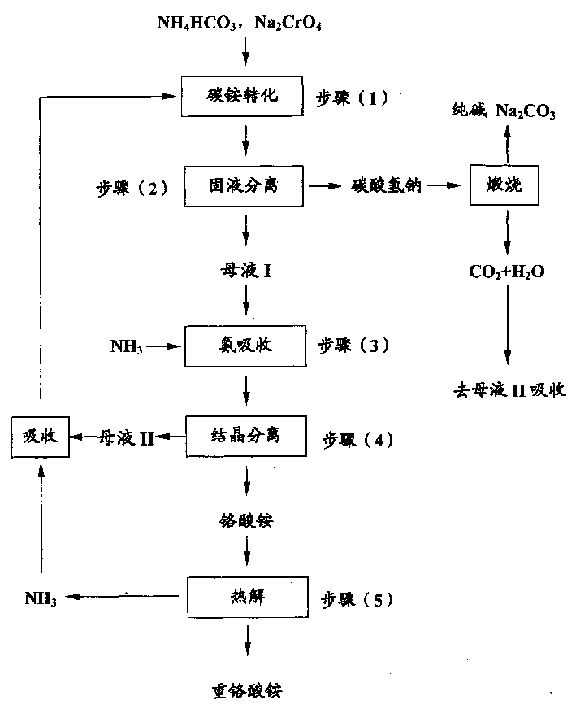
Process for preparing ammonium bichromate
A technology of ammonium dichromate and production method, applied in chromate/dichromate and other directions, can solve problems such as pollution and high cost, and achieve the effects of simple process, easy availability of raw materials and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In a four-necked flask that agitator and thermometer are installed, adding concentration is 280g of sodium chromate aqueous solution 280g (containing Na 2 CrO 4 98.0g, 0.605mol), add ammonium carbonate 96g (1.22mol) again, stir 1 hour at 45 ℃, filter, the sodium bicarbonate precipitation washes three times with 60mol moisture, and washing liquid merges in the mother liquor (I), obtains mother liquor ( 1) about 260ml, the precipitate is calcined at 160°C for 1 hour to obtain soda ash Na 2 CO 3 53g, containing 3% by weight of sodium chromate (which can be directly returned to the chromite roasting unit). In addition, the HCO in the mother liquor (I) was measured 3 - The concentration is about 2.85M, which is HCO 3 -The total content of 0.74mol. Pass into 20g NH in the mother liquor (I) 3 (1.18mol)(NH 3 / HCO 3 - = 1.5 mol / mol). Cool to 16°C to obtain 22.8g of ammonium chromate crystals, dry at 110°C for 2 hours to obtain 19.0g of photographic grade ammonium d...
Embodiment 2
[0031] In a four-necked flask equipped with stirrer and thermometer, adding concentration is 39% by weight of sodium chromate aqueous solution 540g (containing Na 2 CrO 4 211g, 1.30mol), add ammonium bicarbonate 220g (2.78mol) again, stir 0.5 hour at 50 ℃, filter, the sodium bicarbonate precipitate washes three times with 120ml water, and washing liquid merges in the mother liquor (I), obtains mother liquor ( 1) about 500ml. The precipitate was calcined at 120°C for 2 hours to obtain soda ash Na 2 CO 3 155g, containing 8% by weight of sodium chromate (can be directly returned to the chromite roasting unit). In addition, the HCO in the mother liquor (I) was measured 3 - The concentration is 2.96M, i.e. the HCO of the mother liquor (I) 3 - The total content is 1.48mol. Feed 25g NH into the mother liquor 3 (1.47mol)(NH 4 / HCO 3 - =0.99 mol / mol). After cooling to 18°C, 45.6 g of ammonium chromate crystals were obtained, and after drying at 100°C for 2.5 hours, 37.8 ...
Embodiment 3
[0032] In a four-necked flask equipped with a stirrer and a thermometer, adding a concentration is 30% by weight of sodium chromate aqueous solution 300g (containing Na 2 CrO 4 90g, 0.556mol), add ammonium bicarbonate 80g (1.01mol) again, stir 1 hour at 30 ℃, filter, the sodium bicarbonate precipitation washes three times with 90ml water, and washing liquid merges in the mother liquor (I), obtains mother liquor ( 1) about 310ml. Calcining the precipitate at 190°C for 0.5 hours to obtain soda ash Na 2 CO 3 51g, which contains 4.5 weight of sodium chromate (can be directly returned to the chromite roasting unit). In addition, the HCO in the mother liquor (I) was measured 3 - The concentration is 2.27M, i.e. the HCO of the mother liquor (I) 3 - The total content is 0.70 mol. Feed 25g NH into the mother liquor 3 (1.47mol)(NH 4 / HCO 3 - = 2.1 mol / mol). After cooling to 13°C, 24.2 g of ammonium chromate crystals were obtained, and after drying at 80°C for 3 hours, 20....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com