Nicardipine hydrochloride controlled-release preparation
A technology of nicardipine hydrochloride and nicardipine hydrochloride, which is applied in the field of medicine, can solve the problems of frequent doses, troubles for patients, and short intervals, etc., and achieve the effects of reducing the number of doses, improving compliance, and lasting and stable blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
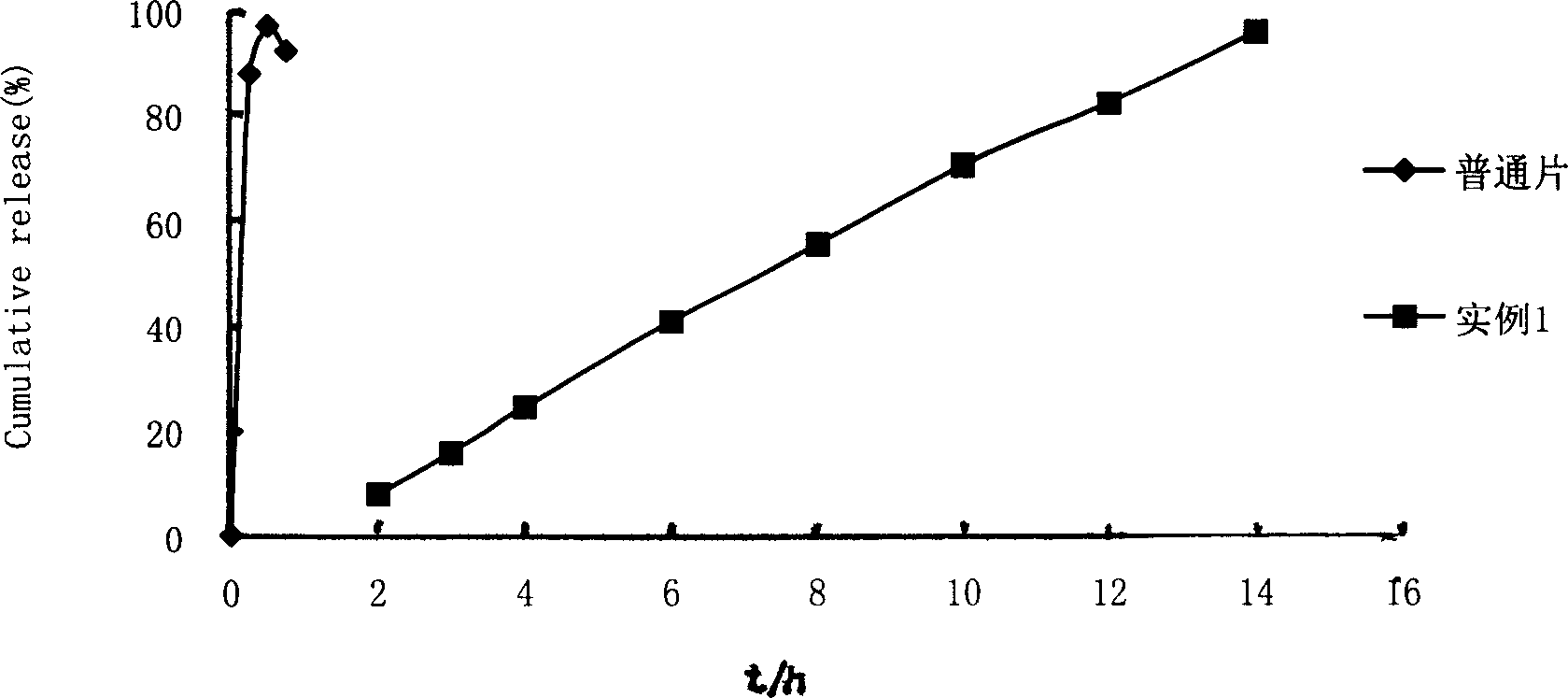
Embodiment 1
[0015] Using film coating technology, nicardipine hydrochloride is made into double-layer osmotic pump controlled-release tablets, and laser drilling is performed on one side to achieve the purpose of controlled release. Present embodiment 1 (composition is calculated by tablet core weight percentage) contains following composition:
[0016] Nicardipine Hydrochloride 14.2%
[0017] Polyoxyethylene N80 31.7%
[0018] Poloxamer 10.7%
[0019] Appropriate amount of talcum powder
[0020] Magnesium Stearate Appropriate amount
[0021] 10% povidone K30 ethanol solution appropriate amount
[0022] Boost layer:
[0023] Polyoxyethylene WSR303 24.5%
[0024] Hypromellose 1.2%
[0026] Povidone 10.7%
[0027] Iron oxide red amount
[0028] Magnesium Stearate Appropriate amount
[0029] 10% povidone K30 ethanol solution appropriate amount
[0030] Coating layer:
[0031] Composition of semi-permeable membrane coating solution (for every 1000 ta...
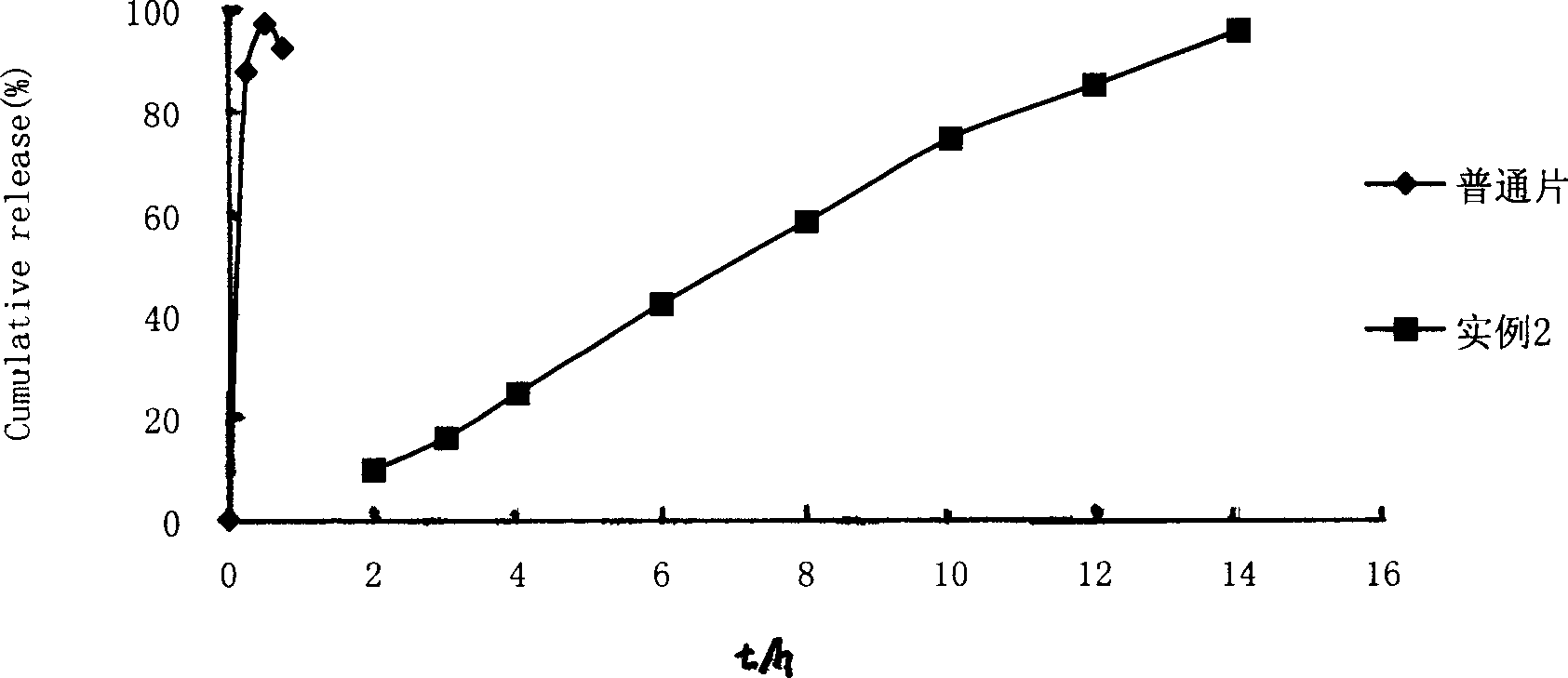
Embodiment 2
[0043] Adopt film coating technology with example 1. Present embodiment 2 (composition is calculated by tablet core weight percentage) contains following composition:
[0044] Drug layer:
[0045] Nicardipine Hydrochloride 14.2%
[0046] Polyoxyethylene N10 31.7%
[0047] Poloxamer 10.7%
[0048] Appropriate amount of talcum powder
[0049] Magnesium Stearate Appropriate amount
[0050] 10% povidone K30 ethanol solution appropriate amount
[0051] Boost layer:
[0052] Polyoxyethylene WSR303 24.5%
[0053] Hypromellose 1.2%
[0055] Povidone 10.7%
[0056] Iron oxide red amount
[0057] Magnesium Stearate Appropriate amount
[0058]10% povidone K30 ethanol solution appropriate amount
[0059] Coating layer:
[0060] Composition of semi-permeable membrane coating solution (for every 1000 tablets)
[0061] Cellulose acetate 25g
[0062] Polyethylene glycol (PEG4000) 0.85g
[0063] Acetone: water 805ml
[0064] Composition of moistu...
Embodiment 3
[0072] Adopt film coating technology with example 1. Present embodiment 3 (composition is calculated by tablet core weight percentage) contains following composition:
[0073] Drug layer:
[0074] Nicardipine Hydrochloride 14.2%
[0075] Hypromellose 31.7%
[0076] Poloxamer 10.7%
[0077] Appropriate amount of talcum powder
[0078] Magnesium Stearate Appropriate amount
[0079] 10% povidone K30 ethanol solution appropriate amount
[0080] Boost layer:
[0081] Polyoxyethylene WSR303 24.5%
[0082] Hypromellose 1.2%
[0084] Povidone 10.7%
[0085] Iron oxide red amount
[0086] Magnesium Stearate Appropriate amount
[0087] 10% povidone K30 ethanol solution appropriate amount
[0088] Coating layer:
[0089] Composition of semi-permeable membrane coating solution (for every 1000 tablets)
[0090] Cellulose acetate 25g
[0091] Polyethylene glycol (PEG4000) 0.85g
[0092] Acetone: water 805ml
[0093] Composition of moisture-pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com