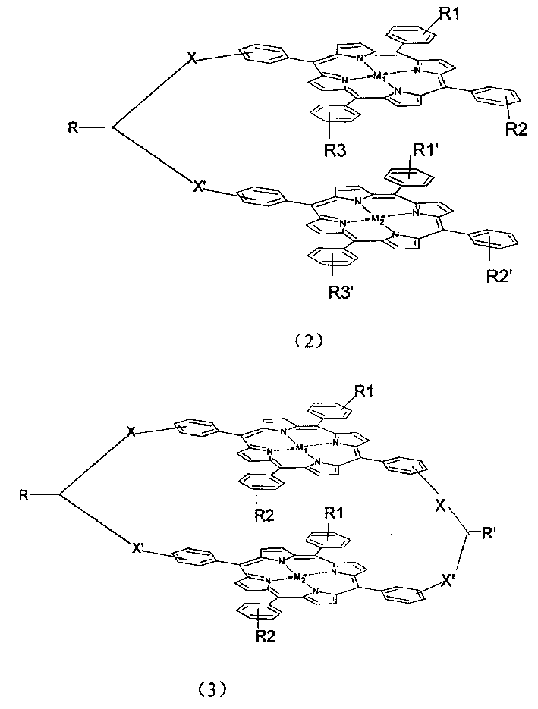
Biporphin metal coordination compound with bridge linking face-face structure and its use
A metal coordination and compound technology, which is applied in the bridging surface structure double porphyrin metal coordination compound and its application field, can solve the problems of unclear mechanism, insufficient resources, high price, etc., and achieve the expansion of the scope of use and the scope of application Wide, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of meso-(5-p-nitrophenyl-10,15,20-triphenyl)porphyrin.
[0049] In a 1000ml three-neck flask equipped with a thermometer, a dropping funnel, a reflux condenser, and a stirrer, add 300-500ml of refined perchloroform, cool in an ice-water bath, and keep the temperature below 1-5°C. 3.00 g (4.875 mmol) of tetraphenylporphyrin was dissolved in chloroform, and the solution was dark red. Add 3.2ml (81.0mmol) of fuming nitric acid dropwise, and finish dropping within 20 to 40 minutes. During this process the solution turned green and gradually darkened to dark green. React for 3-5 hours, neutralize the pH value with ammonia water to 7. The solution turned from dark green to red. The organic phase was separated and washed three times with water. Add anhydrous magnesium sulfate to dry, let stand overnight. Transfer to a distillation flask. Concentrate to 25ml and add methanol 40ml. After standing overnight, 2.40 g of reddish-brown crystals were obta...
Embodiment 2
[0050] Embodiment 2: Preparation of middle-(5-p-aminophenyl-10,15,20-triphenyl)porphyrin
[0051] In a 250ml three-necked flask equipped with a thermometer, a reflux condenser and an electric stirrer, add 50-100ml of concentrated hydrochloric acid. Add 1-5 g of mononitrotetraphenylporphyrin under the protection of argon (or nitrogen). The solution turned from colorless to yellow and then to brown. Dissolve 1-4g of stannous chloride in 10-50ml of concentrated hydrochloric acid, and drop it into the three-necked flask within 5-20min. A large amount of green foam is generated after stirring, and a defoamer is added to defoam. After reacting for 1-4 hours, raise the temperature of the water bath to 60-80°C, keep it for 20-70min, and the color turns dark green.
[0052] Cool the solution, pour it into 80-160ml of ice-water mixture, adjust the pH to 7-8 with ammonia water, the solution turns brown, let stand, and filter with suction to obtain a blue-black solid, which is dried in...
Embodiment 3
[0053] Embodiment 3: Synthesis of middle-(5-p-hydroxyphenyl-10,15,20-three-p-methoxyphenyl)porphyrin
[0054] Add 40-80ml of C2-C6 fatty acid (optimally propionic acid and butyric acid) and 30-60ml of oxidizing benzene with a high boiling point into a 500ml three-necked flask equipped with a stirrer, water separator, and reflux condenser. Derivatives (the best being nitrobenzene) are heated and refluxed for 10-60 minutes, and C2-C6 of p-hydroxybenzaldehyde and p-methoxybenzaldehyde in a molar ratio of 1:3 (total 40 mmol) are added dropwise within 5-20 minutes. Fatty acid solution, add 40mmol (5.36g) freshly steamed pyrrole dropwise within 10-30min, the solution changes from yellow to purple, brown, brown to black. Heat to reflux for 20-80 minutes to obtain a black solution, let it stand and cool overnight, and filter with suction to obtain a black powder. Wash with secondary water and absolute ethanol to obtain blue-black crystals.
[0055] The purification of the crude prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com