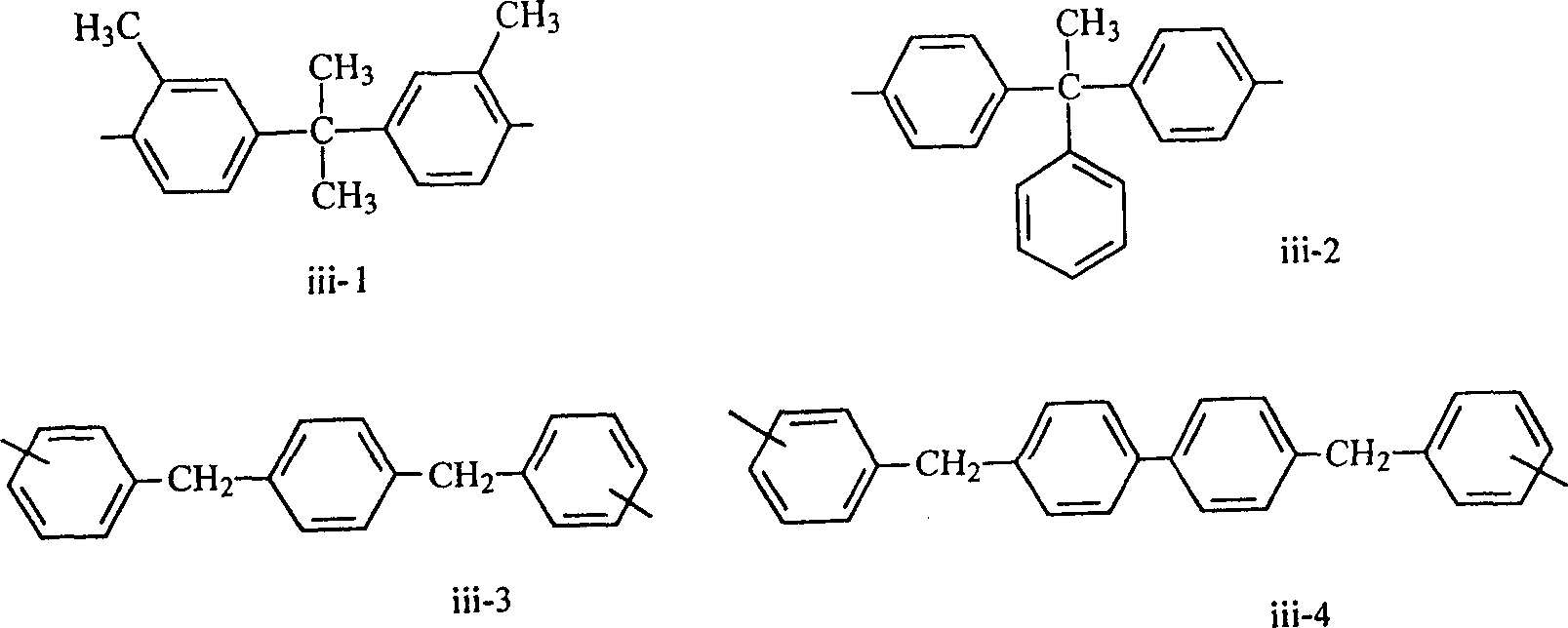
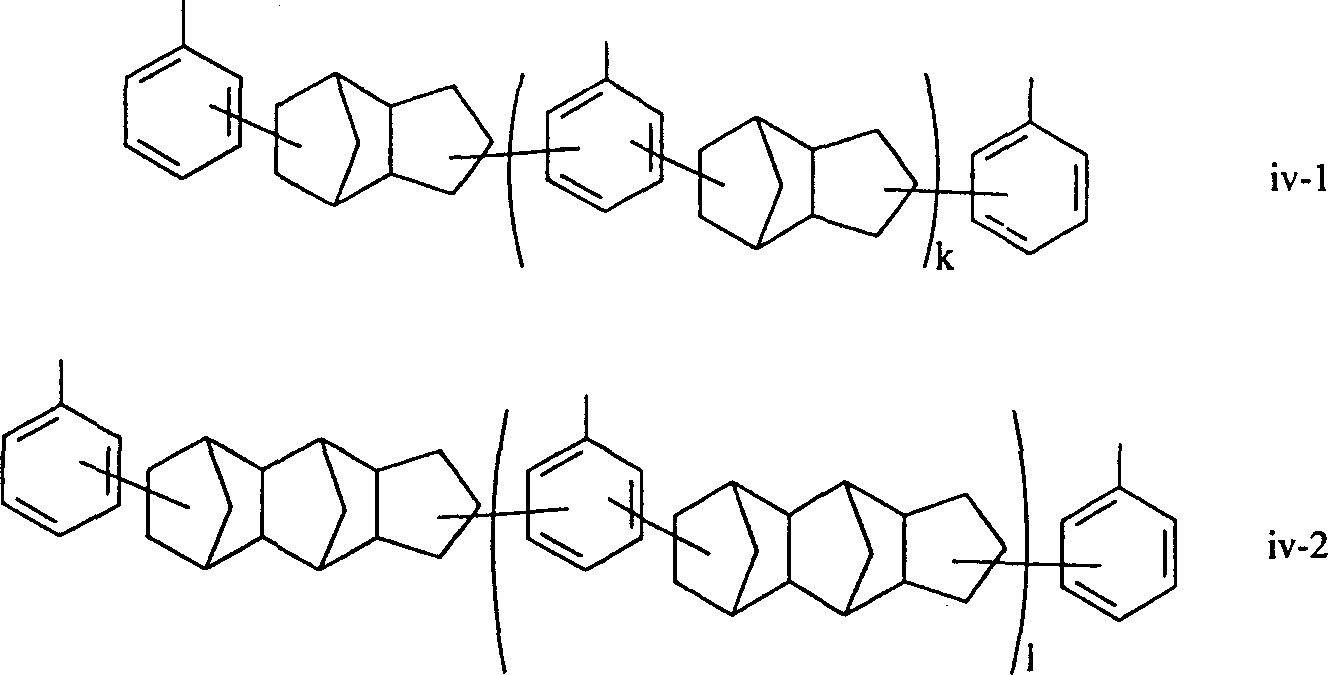
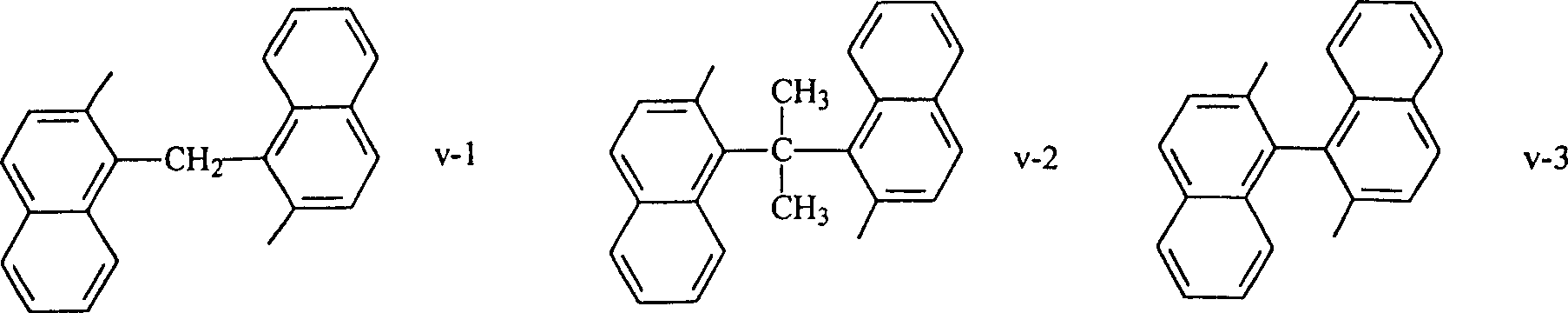
Epoxy resin composition
A technology of epoxy resin and composition, applied in the direction of electrical components, printed circuits, circuit substrate materials, etc., can solve the problems that the end group cannot provide cross-linking, poor heat resistance, low cross-linking density, etc., and achieve excellent Effects of flame retardancy, good solder crack resistance, and low dielectric loss factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0098] 1000 ml of water and 20 g of sodium hydroxide were put into a reaction vessel, and the aromatic monohydroxy compounds and aromatic polyhydroxy compounds in the amounts shown in the column 1 of Synthesis Example 1 in Table 1 were put in a nitrogen gas stream. A Pfaudler impeller was stirred at 300 revolutions per minute for 1 hour. Then, the solution which melt|dissolved the acid halide of the aromatic polyhydric carboxylic acid of the quantity shown in Table 1 in 1000 ml of methylene chloride was dripped into the reaction container maintained at 30 degreeC over 15 seconds, and stirring was continued for 4 hours. The obtained mixed solution was allowed to stand for liquid separation, the aqueous phase was removed, the residual methylene chloride phase was washed with a 0.5% concentration of sodium hydroxide aqueous solution, and the aqueous phase was removed, and the process was repeated 3 times. Water washing and removal of the aqueous phase. After concentrating the wa...
Synthetic example 2~9
[0100] From the raw material compositions of Tables 2 to 3, it was synthesized in the same manner as in Synthesis Example 1 to obtain polyesters (A2) to (A8) and an ester compound (A9).
Synthetic example 10
[0102]400ml of tetrahydrofuran was put into the reaction vessel, 11g of triethylamine and 5.1g of resorcinol were dissolved in a nitrogen stream, and 5.1g of isophthaloyl chloride was dissolved in 100ml by dropwise addition over 30 minutes while cooling with ice. solution in tetrahydrofuran. After stirring for 4 hours, a solution of 19.9 g of p-acetoxybenzoyl chloride dissolved in 100 ml of tetrahydrofuran was added dropwise. After the dropwise addition, the solution was poured into a 5% sodium carbonate aqueous solution, and the precipitate was filtered off with suction, washed with water and methanol, and dried under reduced pressure to obtain a polyester (H1) represented by the following structural formula (in terms of polystyrene). The number average molecular weight of 2900).
[0103]
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com