Kelarmycin dry suspensoid and its preparing process
A technology of clarithromycin and dry suspension is applied in the field of clarithromycin dry suspension and preparation thereof, and can solve the problems of inconvenience in taking, cannot better ensure that patients take medicine according to doctor's orders, and inconvenient for patients to take medicine, Achieve fast onset, good tolerability characteristics, good taste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
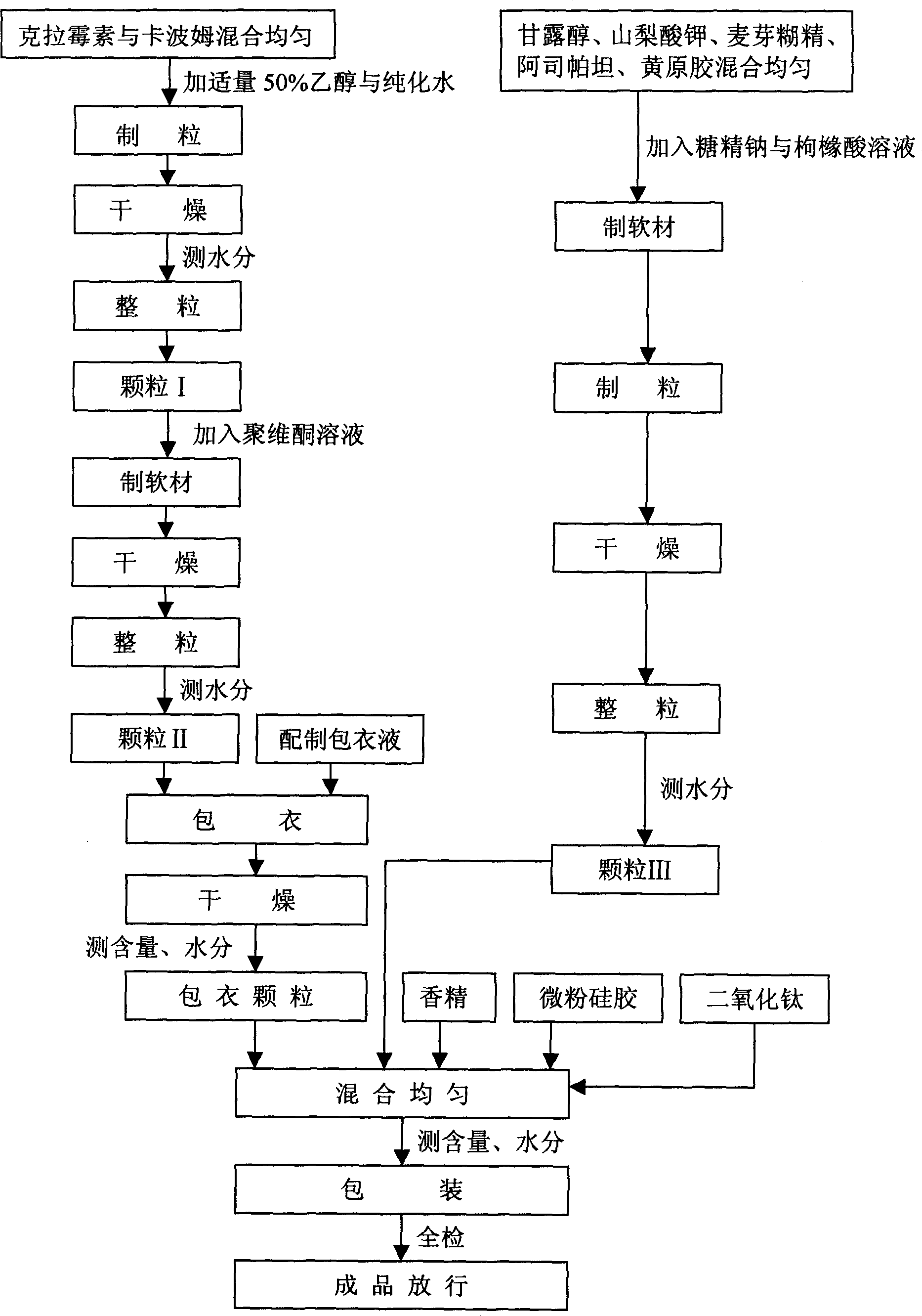
[0031] Please refer to Fig. 1, the present invention also provides the preparation method of this clarithromycin dry suspension, it comprises the following steps:
[0032] 1. Accurately weigh clarithromycin (over 100 mesh), carbomer or (and) povidone, mix evenly, and add an appropriate amount of 50% ethanol to granulate.
[0033] 2. Dry at 40-60°C for about 1-2.5 hours, take a sample to measure the moisture.
[0034] 3. Use a 60-80 mesh sieve for sizing (granule I).
[0035] 4. Add 10-20% carbomer or povidone binder to granule I to make soft material.
[0036] 5. Dry in an oven at 60-70°C for about 4-6 hours.
[0037] 6. Use a 40-60 mesh sieve to sieve the granule, take a sample to measure the water content, and use it as the granule to be coated (granule II).
[0038] 7. Coat the granule II with the hydroxypropylmethylcellulose phthalate coating solution, and dry the coated granule in an oven at 60°C for about 5 hours to obtain the coated granule. Take a sample to measure ...
Embodiment 1
[0052] Clarithromycin dry suspension is characterized in that it consists of the following materials by weight: 6.25 parts of clarithromycin, 3.75 parts of carbomer, 0.875 parts of povidone, hydroxypropyl methylcellulose phthalate 7.6 parts, 0.8 parts of castor oil, 1.775 parts of titanium dioxide, 0.7 parts of xanthan gum, 58.375 parts of mannitol, 0.575 parts of potassium sorbate, 1 part of banana essence, 15 parts of maltodextrin, 1.75 parts of aspartame, 1 part of micronized silica gel parts, 0.3 parts of sodium saccharin, and 0.25 parts of citric acid.
[0053] The film coating material hydroxypropyl methylcellulose phthalate is 5-10 parts by weight.
Embodiment 2
[0055] The product prescription of 125mg clarithromycin dry suspension of the present invention is:
[0056] Raw material name %
[0057] clarithromycin 6.25
[0058] Carbomer or povidone 3-10
[0059] Hypromellose 5-15
[0060] Phthalates
[0061] Xanthan gum 0.5-1.5
[0062] Mannitol 50-60
[0063]Maltodextrin 10-20
[0064] Micronized silica gel 1%
[0065] Citric acid 0.2-1.0%
[0066] 100%
[0067] Film coat: hypromellose phthalate, content: 5-10%.
[0068] The invention uses conventional equipment, has simple preparation process, good taste effect and stable quality. Compared with common preparations, the drug has a quick effect, is delicious to take, is especially suitable for children, and has good tolerance characteristics, so the treatment plan will be simple. For patients, it provides a new treatment option, so developing a dry suspension of clarithromycin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com