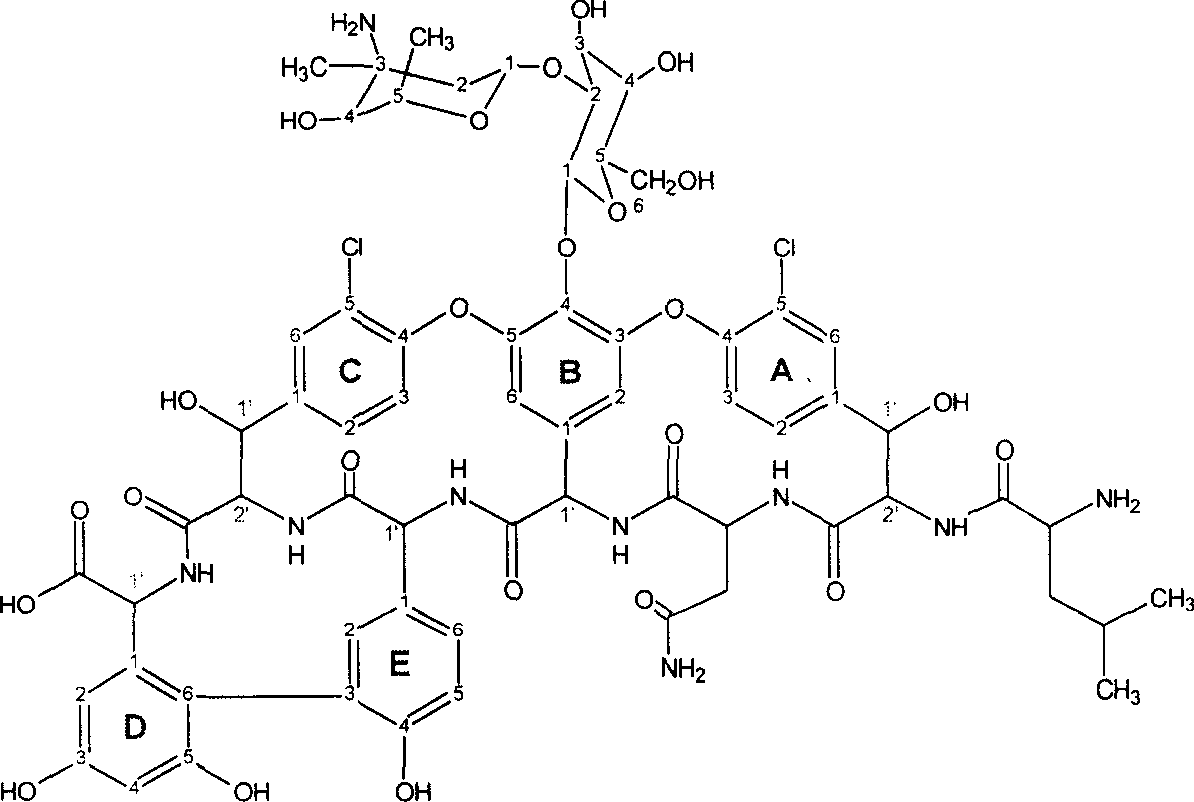
Method for preparing vancomycin of norhydrochloric acid
A methyl vancomycin and hydrochloric acid technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of norvancomycin degradation, poor appearance quality, long filtration time, etc. The effect of production cycle, reduced damage, and improved product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
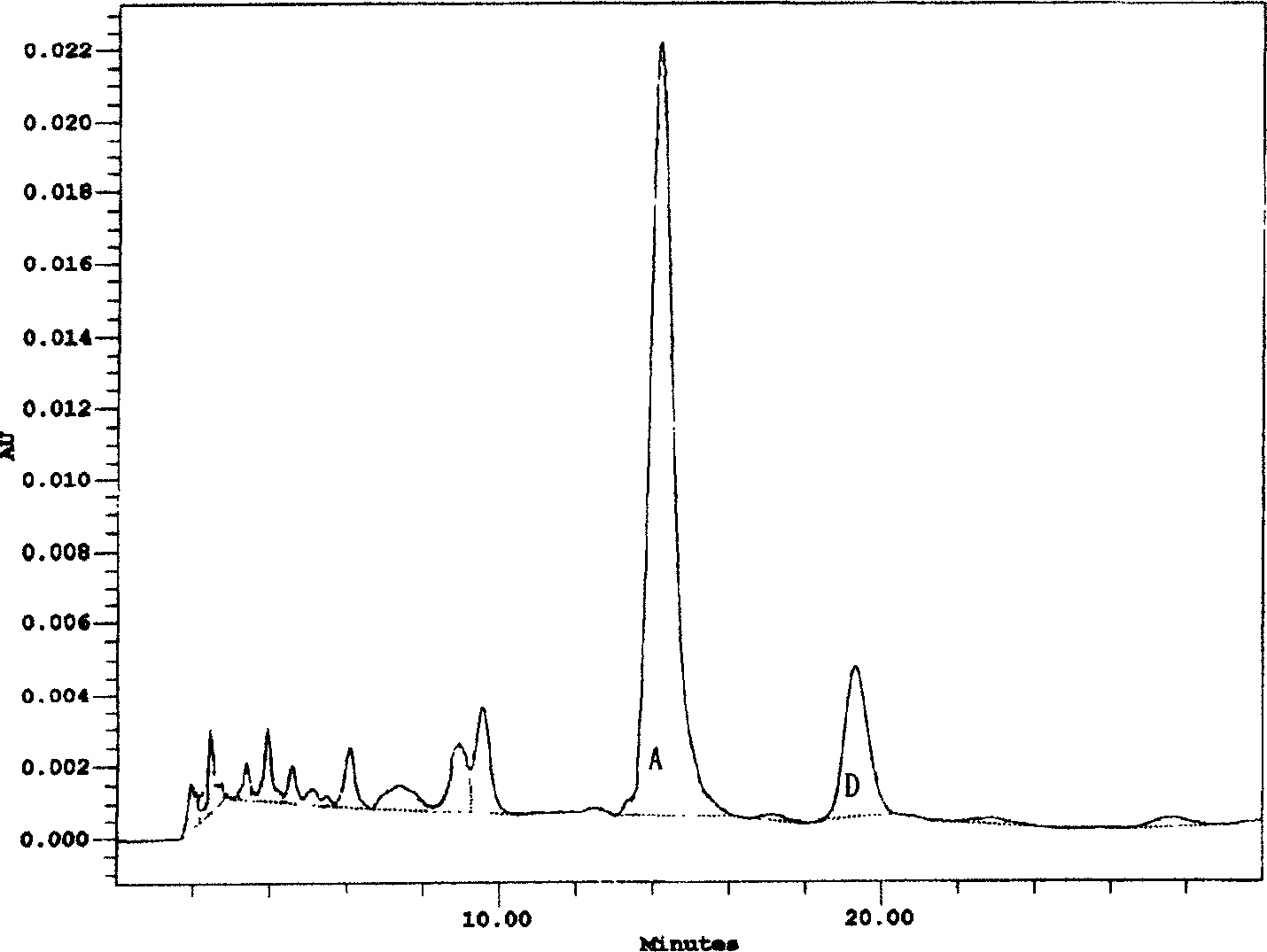
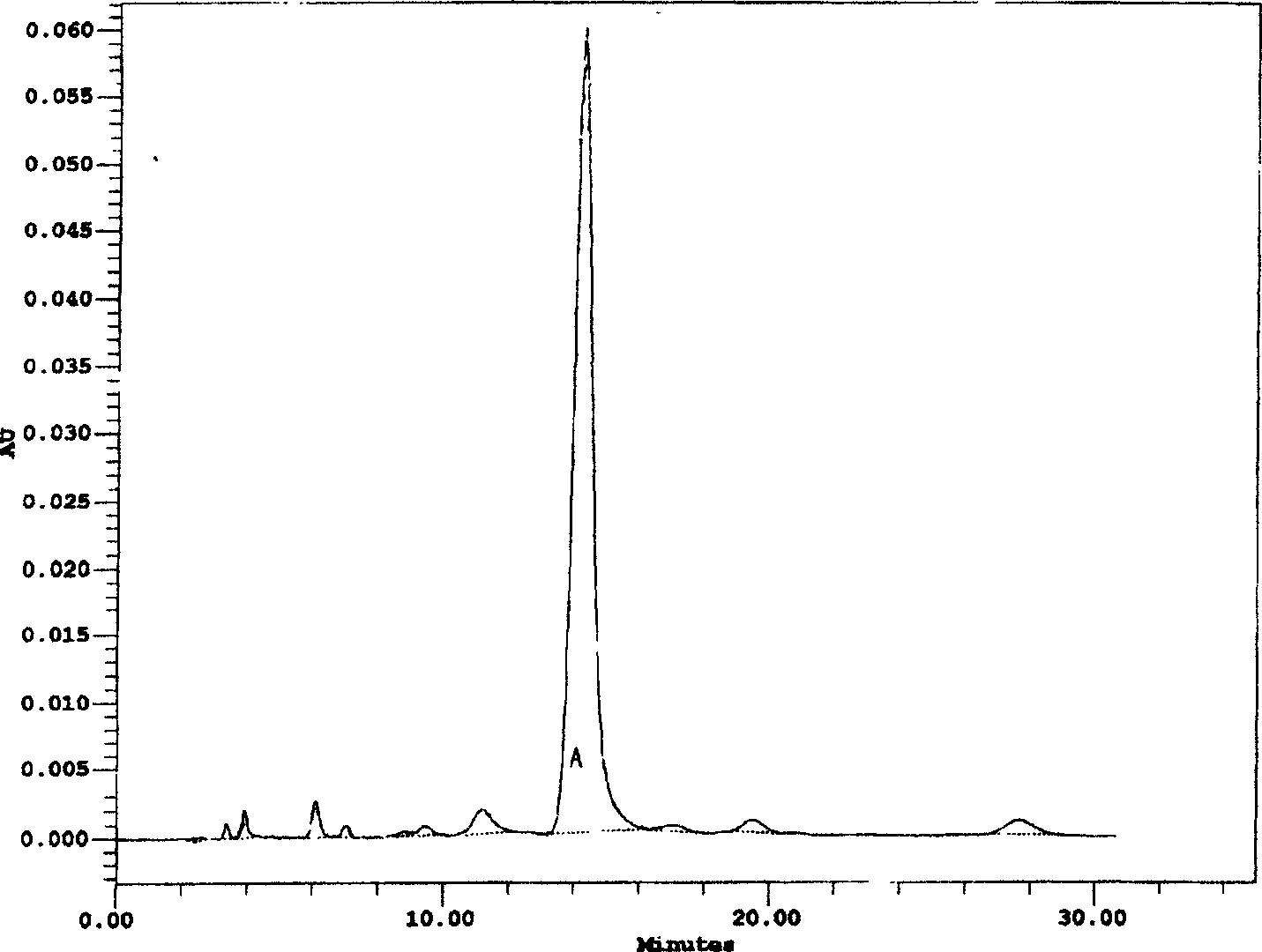
[0028] Preparation of norvancomycin hydrochloride
[0029] Add 1~5% perlite (Henan Zhongnan Filter Aid Co., Ltd.), 2~13% potassium ferricyanide (commercially available product) and 2~10% zinc sulfate (commercially available) to norvancomycin fermented liquid product), stir evenly, plate and frame filter, and the filtrate passes through the column of macroporous decolorizing resin X-5 (Nankai University Resin Factory) with a flow rate of 1~2BV / hour, the unit of decolorizing solution is 3000~5000ug / ml, it is concentrated to 20000~30000ug / ml, the concentrated solution passes through the column of Sephadex G-10 resin (Nankai University Resin Factory) at a flow rate of 1~1.5BV / hour, and the saturated Sephadex G-10 resin uses 0.1mol / L Phosphoric acid aqueous solution elution, the elution rate is 0.5 ~ 1BV / hour, when HPLC detects that demethyl vancomycin hydrochloride flows out, start to collect, slowly add 0.5mol / L sodium hydroxide (commercially available product) aqueous solution t...
Embodiment 2
[0031] Preparation of norvancomycin hydrochloride
[0032] With embodiment 1, just the add-on of perlite is 3%, 7% potassium ferricyanide and 5% zinc sulfate, macroporous decolorizing resin is D941 (Nankai University Resin Factory), and the unit of decolorizing solution is 3672ug / ml, Concentrate it to 23500ug / ml, and the concentrated solution passes through the Sephadex G-15 resin column at a flow rate of 1BV / hour, and the saturated Sephadex G-15 resin is eluted with 0.1mol / L phosphoric acid aqueous solution, and the resolution rate 0.5BV / hour, the analytical solution was adjusted to pH 7.0-7.4 with 0.5mol / L sodium hydroxide aqueous solution, left to stand for 24 hours, plate and frame filtered, the filter cake was dissolved with 1% hydrochloric acid aqueous solution, and 5 times the volume of industrial acetone After crystallization, stand still for 24 hours, plate-and-frame filter press, wash, and vacuum-dry to obtain norvancomycin hydrochloride fine powder. The content of ...
Embodiment 3
[0034] Preparation of norvancomycin hydrochloride
[0035] With embodiment 1, just the add-on of perlite is 5%, 13% potassium ferricyanide and 2% zinc sulfate, use XAD-III macroporous decolorizing resin (Nankai University Resin Factory) decolorizing, the unit of decolorizing liquid is 4120ug / ml, it is concentrated to 27800ug / ml, the concentrated solution passes through the Sephadex G-25 resin column at a flow rate of 1BV / hour, and the saturated Sephadex G-25 resin is analyzed with 0.1mol / L hydrochloric acid aqueous solution, The analysis speed is 0.5BV / hour, the analysis solution is adjusted to pH 7.8 with 0.5mol / L sodium hydroxide aqueous solution, let stand for 24 hours, plate and frame filter, the filter cake is dissolved with 1% hydrochloric acid aqueous solution, and 10 times the volume of industrial acetone After crystallization, stand still for 24 hours, plate and frame press filter, wash, and vacuum-dry to obtain norvancomycin hydrochloride fine powder. The content o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com