Ultraviolet absorber for synthetic resin and synthetic resin composition containing the same
A synthetic resin, ultraviolet technology, applied in the direction of composition, organic chemistry, other chemical processes that inhibit chemical changes, etc., can solve the problems of turbidity, transparency, whitening, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0114] Hereinafter, the present invention will be described in more detail through production examples, examples, and comparative examples. However, the present invention is not limited in any way by these Examples and the like.
Synthetic example
[0116] Synthesis of intermediate compounds
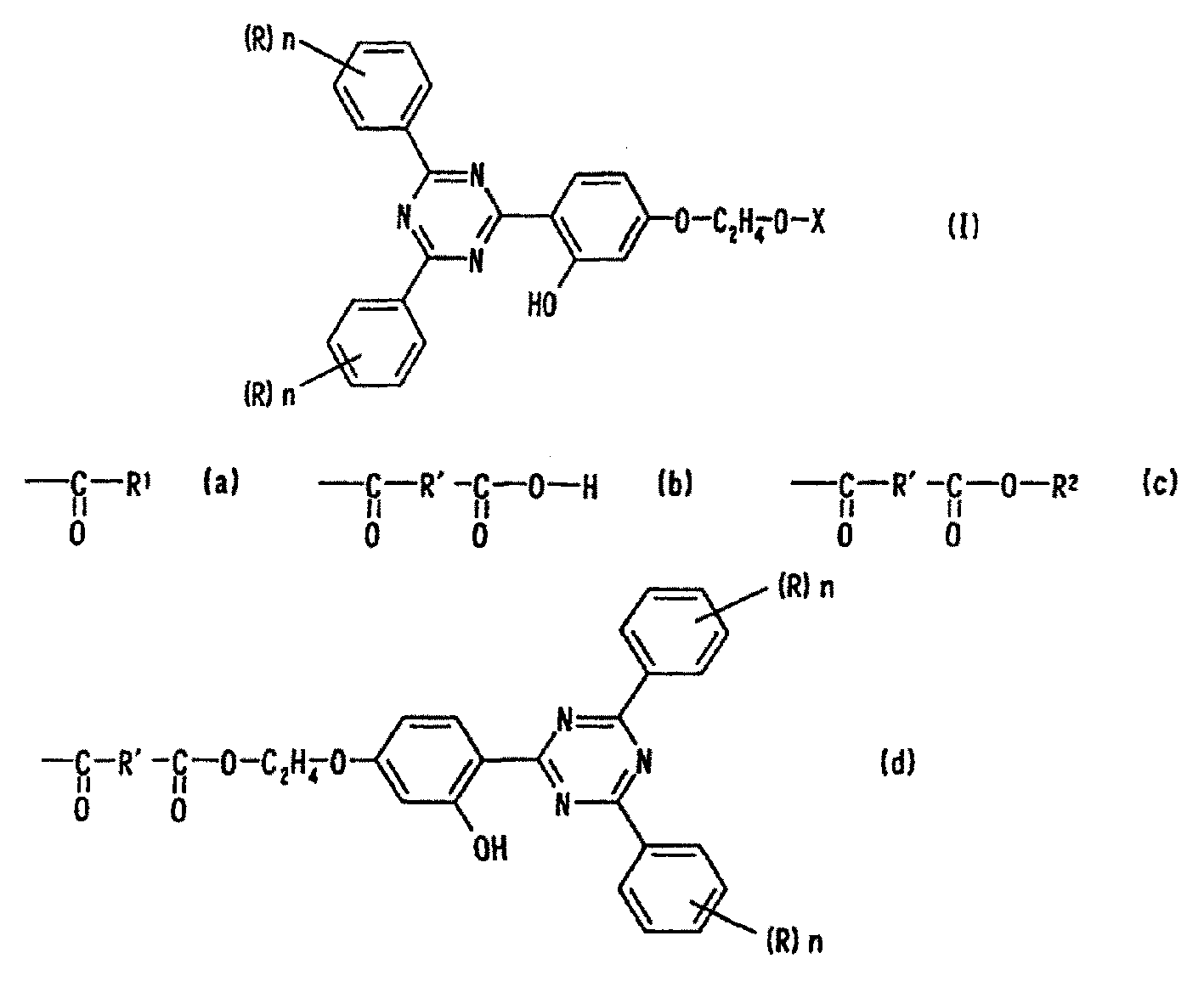
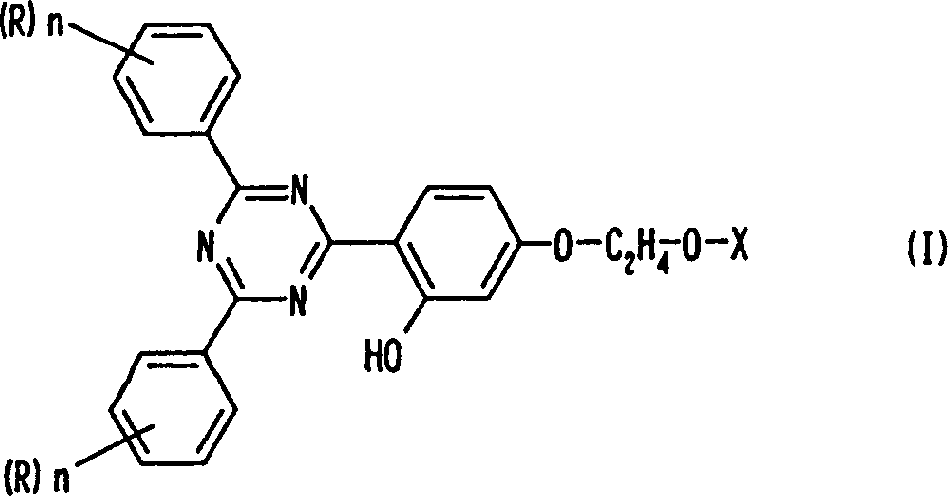
[0117] In the reaction flask, 141 grams of benzamidine hydrochloride and 99.0 grams of resorcinol benzoate were dissolved in 580 grams of ethanol, 175 grams of methanol solution of 28% by mass sodium methoxide was added, and the mixture was heated at 78°C while stirring. Methanol was removed. After stirring at 78°C for 20 hours, it was cooled to 5°C, and the precipitated solid phase was collected by filtration. The resulting solid phase was washed with methanol and water to obtain 55.2 g of intermediate 2-(2,4-trihydroxyphenyl)-4,6-diphenyl-1,3,5-tri Zine (yield 38%).
[0118]Add 51.0 grams of 2-(2,4-trihydroxyphenyl)-4,6-diphenyl-1,3,5-triazine, 284 grams of 2-bromoethanol, 255 grams of dimethylformamide, then dripped 87.6 grams of 48 mass % sodium hydroxide aqueous solution at 85°C, reacted at 85°C for 10 hours, cooled to 5°C, and then added dropwise at a temperature below 5°C hydrochloric acid, neutralized to pH 7-6, filtered...
manufacture example 1
[0120] Synthesis of Compound No.1
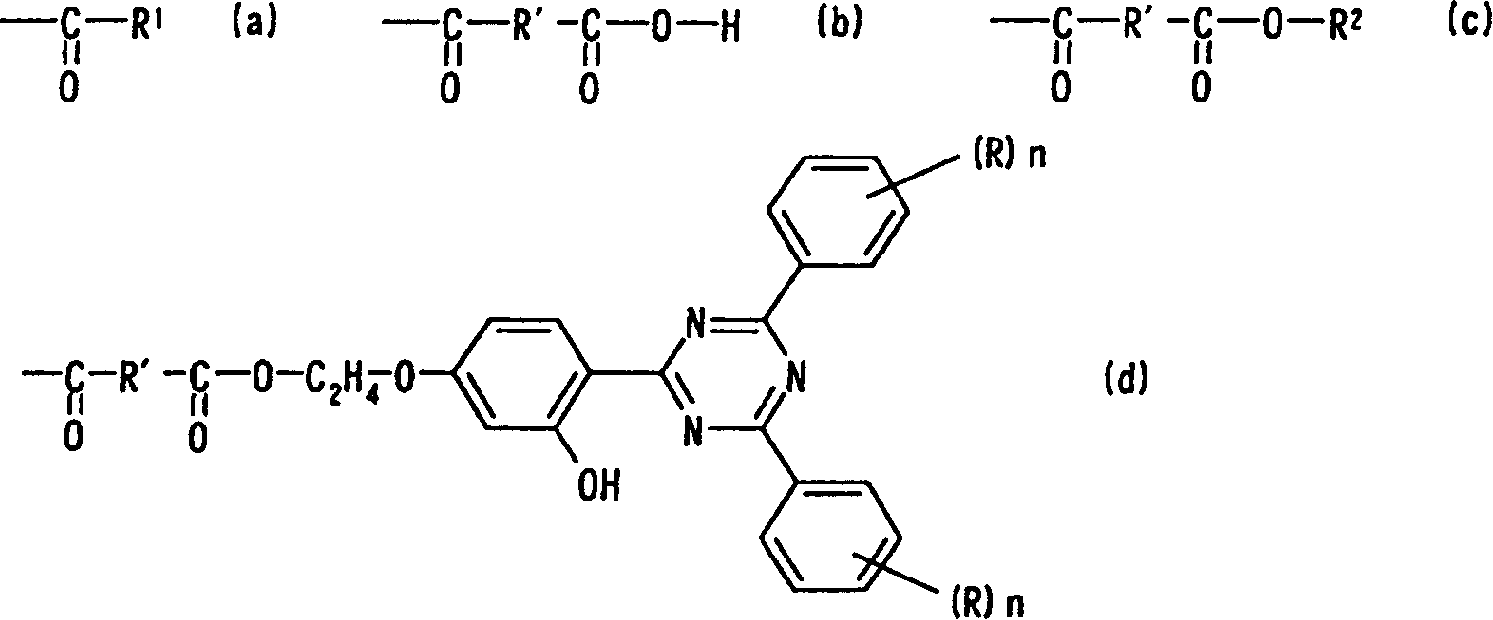
[0121] In the reaction flask replaced by nitrogen, 2.58 grams of cyclohexanecarboxylic acid, 50 grams of xylene, and 0.10 grams of p-toluenesulfonic acid were added to 7.94 grams of the intermediate compound obtained in the above synthesis example, and the mixture was heated at 140 ° C. Dehydration and reflux for 10 hours. Wash with water until the pH value in the system is 7, filter the solid phase after filtration, then add methanol to precipitate crystals, and use xylene / methanol (1 / 3) solvent to recrystallize the crude crystals obtained after filtration to obtain Pale yellow crystals were obtained in a yield of 81%. The obtained crystals were analyzed as follows, and it was confirmed that the crystals were the target compound, that is, Compound No. 1.
[0122] ① Infrared (IR) analysis (cm-1): 3440 (hydroxyl), 2925, 2850 (cyclohexyl), 1725 (ester), 1535, 1510 (triazinyl), 1165 (ether).
[0123] ② Elemental analysis (mass%): carbon; 72....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com