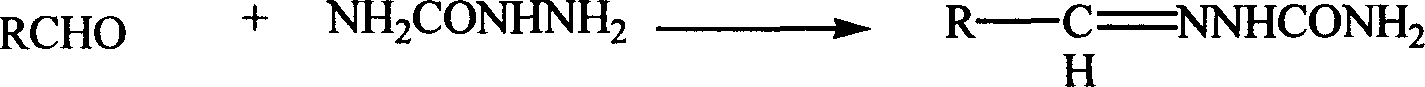Semicarbazone synthesis method
A synthetic method and semicarbazone technology, applied in chemical instruments and methods, preparation of urea derivatives, preparation of organic compounds, etc., can solve the problems of trimethylsilyl isocyanate dependence on imports, low equipment level, and human health hazards. Achieve non-flammable and explosive safety, easy operation and handling, and beneficial to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 is synthesized in the benzaldehyde semicarbazone in ionic liquid
[0025] Add urea (10g, 0.167mol) and 50% hydrazine hydrate (9g, 0.09mol) successively into a 100mL three-necked round-bottomed flask equipped with a thermometer and a condenser, react at 100°C for 4h, cool to room temperature, and adjust the pH with concentrated hydrochloric acid 3~4, dropwise add the mixed solution of benzaldehyde (9mL, 0.085mol) and 10mL 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, a white precipitate is formed immediately, after the addition, stir at room temperature for 2h , heated to reflux for 1 h, cooled and filtered to obtain white crystals, 12.1 g, yield 95.8%, melting point 220-221 °C. The filtrate was extracted with dichloromethane, and the solvent was evaporated to obtain 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, which was recycled for use.
Embodiment 2
[0026] Embodiment 2 is synthesized in the benzaldehyde semicarbazone under ultrasonic promotion
[0027] Add urea (10g, 0.167mol) and 50% hydrazine hydrate (9g, 0.09mol) successively into a 100mL three-necked round-bottomed flask equipped with a thermometer and a condenser, react at 95°C for 4h, cool to room temperature, and adjust the pH with concentrated hydrochloric acid 3~4, dropwise add the mixed solution of benzaldehyde (9mL, 0.085mol) and 10mL 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, a white precipitate is formed immediately, after the addition, the reaction is promoted by ultrasonic waves 0.5h, heated to reflux for 1h, cooled and filtered to obtain white crystals, 12.2g, yield 96.4%, melting point 219-220°C. The filtrate was extracted with dichloromethane, and the solvent was evaporated to obtain 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, which was recycled for use.
Embodiment 3
[0028] The synthesis of benzaldehyde semicarbazone under the microwave radiation of embodiment 3
[0029] Add urea (10g, 0.167mol) and 50% hydrazine hydrate (9g, 0.09mol) successively into a 100mL three-necked round-bottomed flask equipped with a thermometer and a condenser, react at 100°C for 4h, cool to room temperature, and adjust the pH with concentrated hydrochloric acid 3~4, dropwise add the mixed solution of benzaldehyde (9mL, 0.085mol) and 10mL 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, immediately a white precipitate is formed, after the addition, react under 125W microwave After 10 minutes, cool and filter to obtain white crystals, 12.4 g, yield 98.0%, melting point 219-222°C. The filtrate was extracted with dichloromethane, and the solvent was evaporated to obtain 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, which was recycled for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com