Macroporous zeolite absorbent and preparation method thereof
A large-pore zeolite and adsorbent technology, which is applied in the field of large-pore zeolite adsorbent and its preparation, can solve the problems of affecting diffusion, small average pore size, and reducing the use efficiency of adsorbent, so as to achieve the advantages of diffusion, uniform distribution of coarse pores, The effect of large average pore size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0020] Embodiments 1-4: Preparation of sodium zeolite powder crystals
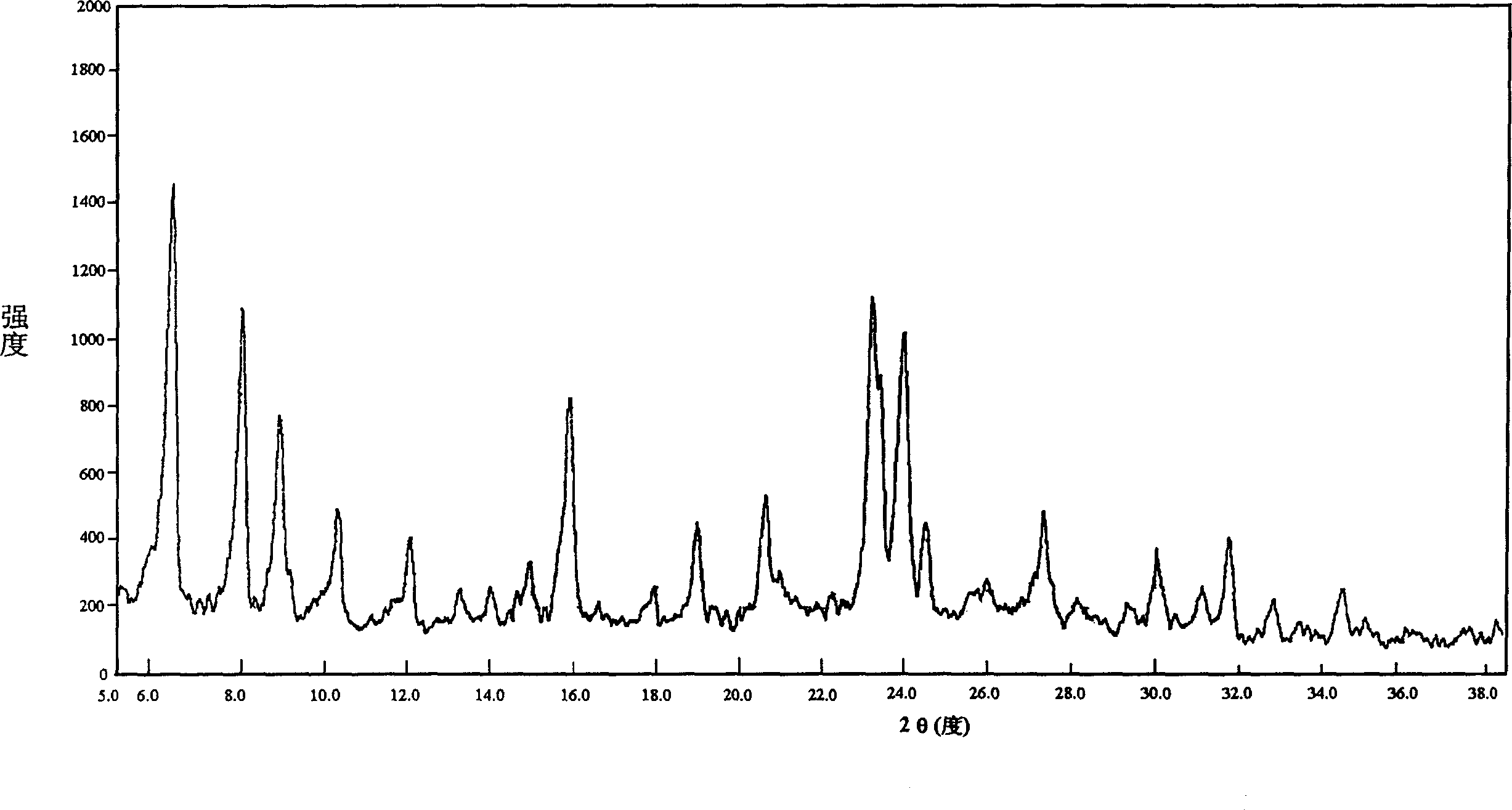
[0021] Water glass is weighed respectively by the reactant molar proportion of table 1 (its mass chemical composition (%): SiO 2 = 25.33; Na 2 O=7.28), solid aluminum sulfate Al 2 SO 4 18H 2 O, sulfuric acid and water, after mixing, vigorously stir for 1 to 2 hours until uniform Na 2 O-SiO 2 -Al 2 o 3 -H 2 O reactant colloid. The colloid is placed in a stainless steel high-pressure reaction kettle, sealed, heated to a set temperature in a constant temperature oven, left to stand at constant temperature for a predetermined time, and taken out of the reaction kettle from the oven and cooled to room temperature. The reaction product was washed with deionized water, filtered and dried. The crystal phase of the synthesized product zeolite was identified by XRD diffractometer. Except containing a small amount of miscellaneous crystals in the product of example 2, other synthetic products are all sodiu...
Embodiment 5~7
[0023] Examples 5-7: Sodium-type zeolite cation-exchange hydrogen-producing zeolite
[0024] Weigh 500 grams of the sodium-type zeolite synthesized in Example 3, and carry out ion exchange with the acid (or ammonium salt) solutions listed in Table 2 in a stainless steel container to obtain the hydrogen-type zeolite. The exchange product was washed with deionized water until pH = 6, filtered and dried. Analyzing the Na of the obtained hydrogen-type zeolite 2 O content, the data are listed in Table 2 to prove that the exchange effect is good.
[0025] Example
Embodiment 8
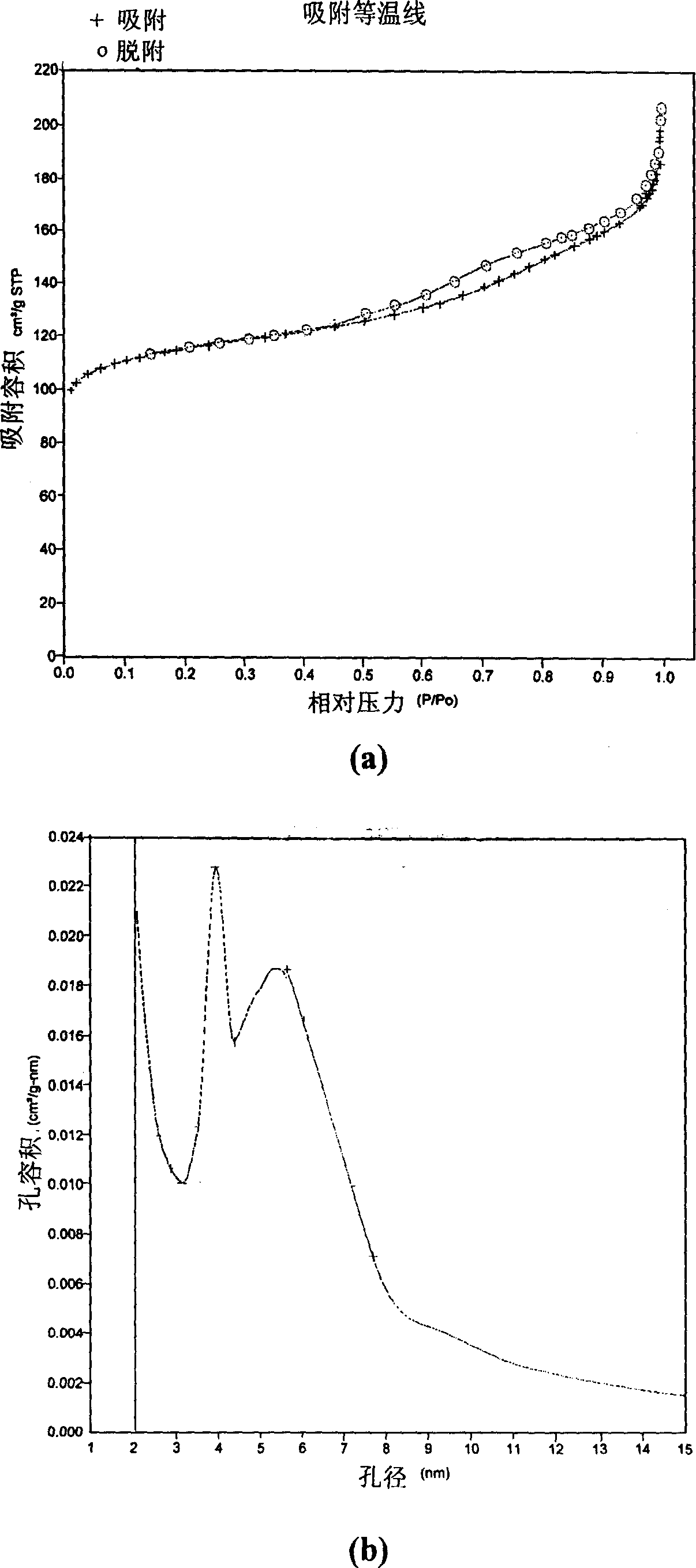
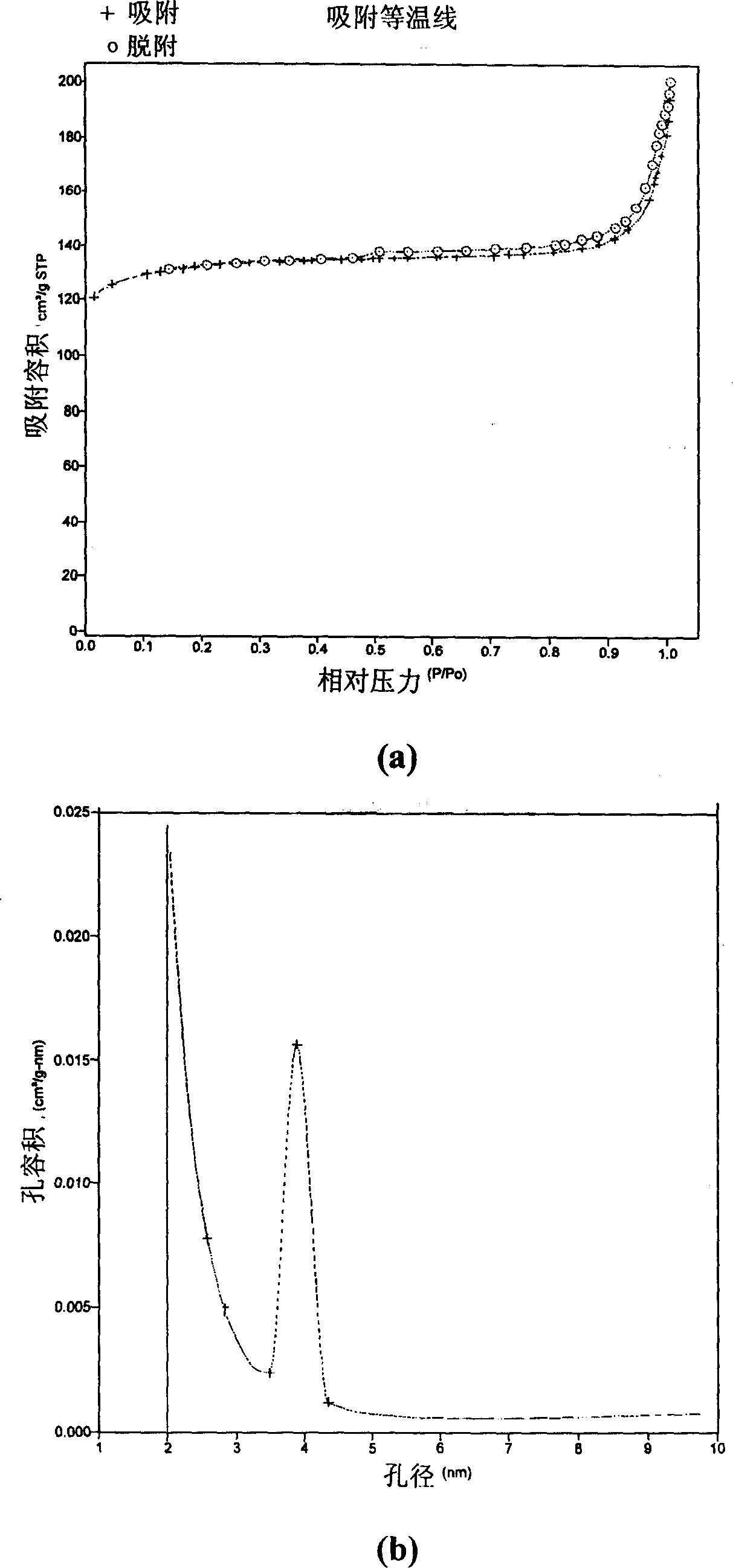
[0027] Use the preparation conditions of Example 6 to scale up and prepare 10 kg of hydrogen-type zeolite powder crystals. In the rolling ball forming machine, powder crystal and silica sol binder (containing 30% SiO 2 ) to make adsorbent pellets with a diameter of 1.5-2.2 mm. Calculated according to the amount of the binder, in the prepared adsorbent pellets, on a dry weight basis, the amorphous silica binder / hydrogen zeolite=0.2. After drying in an oven, the content of silica and alumina was measured, and the molar ratio of silica to alumina was calculated to be 15.5. After the adsorbent was activated at 550°C, its low-temperature nitrogen adsorption isotherm and pore size distribution diagram were measured with an automatic adsorption instrument, and its surface area, pore volume and average pore size were calculated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com