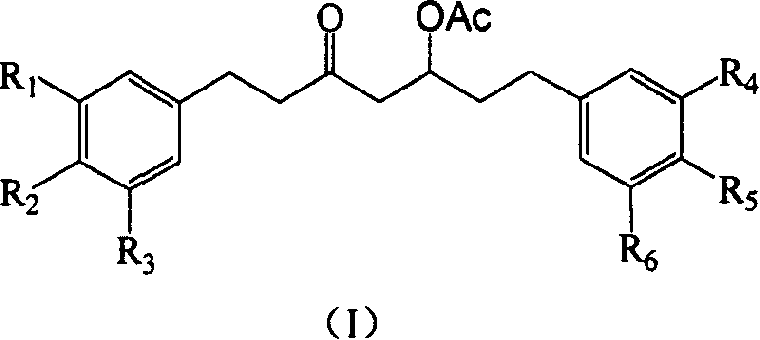
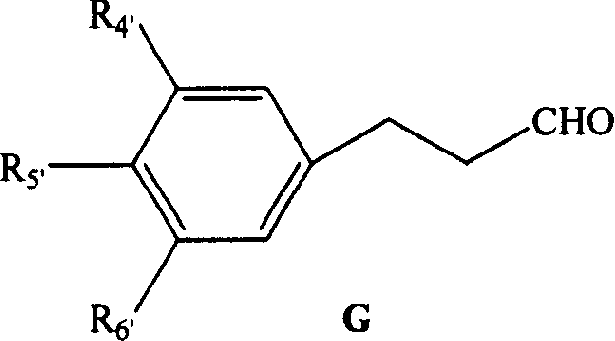
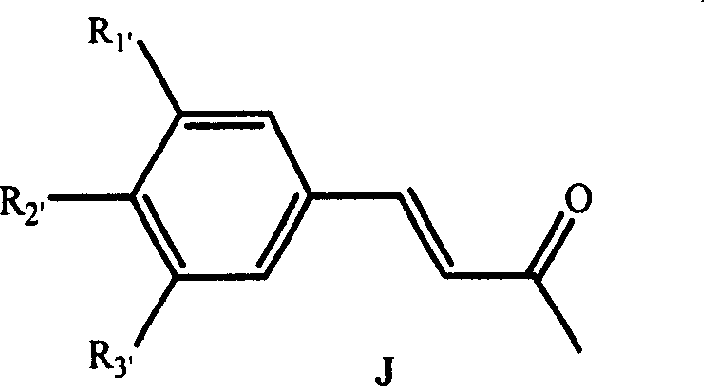
Diphenyl heptanone compound, its production and use
A technology of diphenylheptanone and compounds, applied in the preparation of organic compounds, chemical instruments and methods, and preparation of carboxylic acid esters, can solve the problems of poor patient tolerance, large side effects, curative effect and application impact, etc., and achieve strong Therapeutic effect, significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Synthesis of compound 1 [5-acetoxy-1-(3'-methoxy-4'-hydroxyphenyl)-7-(4"-methoxyphenyl)-3-heptanone]
[0071] The synthetic route of compound 1 is as follows:
[0072]
[0073] Synthesis of 4-methoxycinnamic acid (II):
[0074] 25g (180mmol) of p-methoxybenzaldehyde, 32g of malonic acid and 25ml of pyridine were mixed, and 1ml of hexahydropyridine was added. Stir the reaction at 85-120°C for 6 hours, cool the reactant, add 120g of 25% potassium carbonate solution, stir for 15min, filter, and acidify the filtrate with concentrated hydrochloric acid to PH<1, the crude product is precipitated, and the crude product is recrystallized by ethanol to obtain white Crystal, product 30.4g, yield 95%.
[0075] Synthesis of 4-methoxyphenylpropionic acid (III):
[0076] Dissolve 2.38g (13.4mmol) of compound II in 30ml of methanol, add 0.15g of 5% Pd / C, feed hydrogen, stir at room temperature, check the progress of the reaction by TLC, the reaction is complete after a...
Embodiment 2
[0093] Example 2 Synthesis of Compound 2 [5-Acetoxy-1-(3′,4′-dimethoxy-phenyl)-7-(4″-methoxyphenyl)-3-heptanone]
[0094] The synthetic route of compound 2 is as follows:
[0095]
[0096] The synthesis of 4-methoxyphenylpropan-3-aldehyde (VI) is with embodiment 1.
[0097] 3, the synthesis of 4-dimethoxybenzaldehyde (VIII):
[0098] Dissolve 1.52g (10mmol) of 3-methoxy-4-hydroxybenzaldehyde VII in 30ml of anhydrous acetone, add anhydrous K 2 CO 3 1.8g, stirred vigorously at room temperature, added dropwise CH 3 I (14.0mmol), reacted at room temperature for 4 hours, after the reaction was complete, filtered K 2 CO 3 , 1.50 g of a yellow oily liquid was obtained, and the yield was 90%.
[0099] 3, the synthesis of 4-dimethoxybuten-3-one (IX):
[0100] Dissolve 1.66 g (10 mmol) of compound VIII in 20 ml of acetone, slowly add 25 ml of 1% NaOH in an ice-water bath, then remove the ice bath, react at room temperature, check the progress of the reaction by TLC, complete t...
Embodiment 3
[0106] Example 3 Compound 3[5-acetoxy-1-(3'-methoxy-4'-acetoxy-phenyl)-7-(4"-methoxyphenyl)-3-heptanone ]Synthesis
[0107] The synthetic route of compound 3 is as follows:
[0108]
[0109] The synthesis of 1-(3'-methoxyl-4'benzyloxyphenyl)-7-(4"-methoxyphenyl)-5-hydroxyl-3-ketone-1-heptene (X) is the same as Example 1
[0110] Synthesis of 5-acetoxy-1-(3'-acetoxy-4'-methoxy-phenyl)-7-(4"-methoxyphenyl)-3-heptanone (3):
[0111] Dissolve 223 mg (0.5 mmol) of compound X in 20 ml of anhydrous methanol, add 0.15 g of Pd / C, pass through hydrogen, stir at room temperature for about 24 hours, TLC detects that the reaction is complete, filter under reduced pressure, evaporate the solvent, and the silica gel column layer Analysis, petroleum ether: acetone = 4: 1 elution gave 1149 mg of yellow powdery crystals, yield 83%. Dissolve 358mg (1mmol) in 8ml of pyridine, add 0.5ml of acetic anhydride after complete dissolution, complete reaction after 1 hour, evaporate the solvent to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com