Method for preparing fusion protein contg. human interferon-beta and human seralbumin and its products
A technology of human serum albumin and fusion protein, which is applied in the field of long-acting fusion protein drugs and can solve the problems of reducing the curative effect of fusion proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
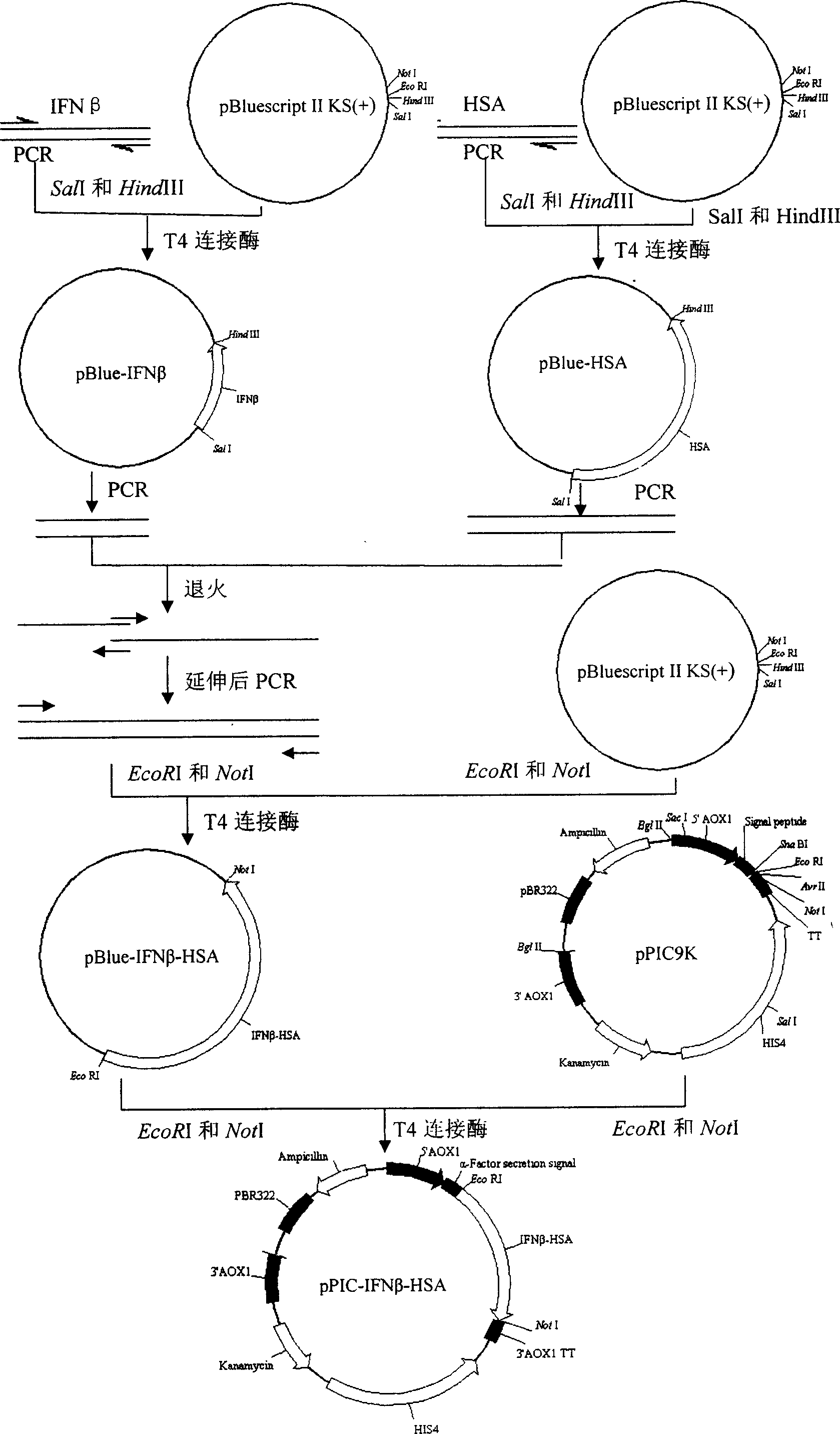
[0024] Example 1: Cloning of IFNβ cDNA
[0025] Whole blood genomic DNA purification system (purchased from Shanghai Bioengineering Technology Service Company) was used to extract genomic DNA from healthy human blood concentrated leukocytes according to the operating instructions. The extracted DNA was treated with restriction endonuclease EcoRI or HindIII, and the cDNA of IFNβ was amplified by PCR, and the primers used were as follows:
[0026] Pb1: 5'-TA GTC GAC ATGAGCTACAACTTGCTTGG-3'
[0027] Pb2: 5'-AG AAGCTT TCAGTTTCGGAGGTAACCTG-3'
[0028] The PCR method is as follows:
[0029] Add to the 50μl reaction system: 3μl of 10μmol / L Pb1 and Pb2 primers, 5μl of 2mmol / L dNTP, 1μl of 10×pfu Buffer, 0.5μl of 5U / μl pfu DNA polymerase (dNTP, 10×pfuBuffer and pfu All DNA polymerases were purchased from Shanghai Bioengineering Technology Service Company, the same below), and 1 μg of human genomic DNA treated with the enzyme was added to 50 μl of double distilled water. PTC-100...
Embodiment 2
[0031] Example 2: Cloning of HSAcDNA
[0032] HSA cDNA was amplified from human fetal liver cDNA library by PCR. The primers used were:
[0033] HSA1: 5'-AGA GTC GAC GATGCACACAAGAGTGAGGTTGCTC-3'
[0034] HSA2: 5'-GCC AAGCTT TTATAAGCCTAAGGCAGCTTGACTT-3'
[0035] The PCR method is as follows:
[0036] Add to the 50 μl reaction system: 3 μl of 10 μmol / L HSA1 and HSA2 primers, 5 μl of 2 mmol / L dNTP, 1 μl of 10×pfu Buffer, 0.5 μl of 5 U / μl pfu DNA polymerase, 1 μg of human fetal liver cDNA library, Add double distilled water to make up 50 μl. PTC-100 at MJ Research TM On the PCR instrument, the PCR conditions are: fully denature at 95°C for 5 minutes, denature at 94°C for 1 minute, anneal at 60°C for 1 minute, extend at 72°C for 90 seconds, and cycle 30 times.
[0037] The reaction product was analyzed by agarose gel electrophoresis, and a band of expected size (about 1.8 kb) appeared in the loading lane. A 1.8 kb target fragment was purified with a PCR Fragment Gel Reco...
Embodiment 3
[0038] Embodiment 3: Cloning of fusion gene of IFNβ cDNA and HSA cDNA
[0039] PCR amplification of IFNβ cDNA:
[0040] The primers used are as follows:
[0041] Pf1: 5'-CCCTCC GAATTC AAAAGAATGAGCTACAACTTGCTTGGA-3'
[0042] Pf2: 5'-ACCTCACTCTTGTGTGCATCGTTTCGGAGGTAACCTGTAA-3'
[0043] The PCR method is as follows:
[0044] Add to the 50μl reaction system: 10μmol / L Pf1 and Pf2 primers 2.5μl each, 2mmol / L dNTP 5μl, 10×pfu Buffer 1μl, 5U / μl pfu DNA polymerase 0.5μl, pBlue-IFNβ 1ng, add Make up 50 μl of double distilled water. PTC-100 at MJ Research TM On the PCR instrument, the PCR conditions are: fully denature at 95°C for 5 minutes, anneal at 65°C for 1 minute, extend at 72°C for 90 seconds, denature at 94°C for 1 minute, and cycle 30 times.
[0045] PCR amplification of HSA cDNA:
[0046] The primers used are as follows:
[0047] Pf3 5'-TTACAGGTTACCTCCGAAACGATGCACACAAGAGTGAGGTT-3'
[0048] Pf4: 5'-CATAAG GCGGCCGC TTATTATAAGCCTAAGGCAGCTTGA-3'
[0049] The PCR metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com